Part
8: THERMODYNAMICS
This should have been a reasonably straight forward calculation, but care
needs to be taken to get the right structures and the right molecular formula,
then do the math correctly.
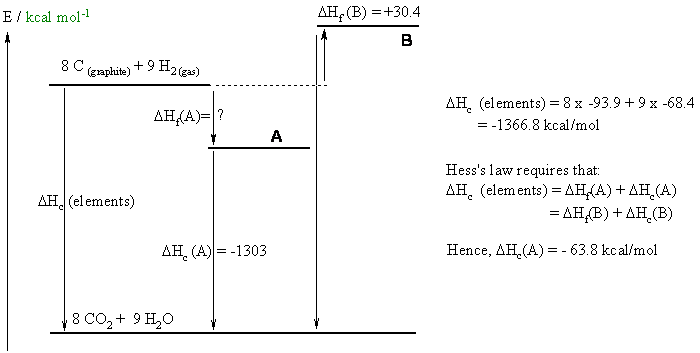
a. C8H18 + 12.5 O2--> 8 CO2 + 9 H2O
b. 
c. Since the more branched isomer (2,2,3,3-tetramethylbutane) is the more stable isomer, it will be the one with the most exothermic heat of formation, i.e. -63.8 kcal/mol. The more branched isomer is more stable because more branches maximises the number of stronger primary C-H bonds.

d. The easiest way to evaluate a heat of reaction is based on the bonds that change since heat of reaction = bonds broken - bonds formed (since it requires energy to break bonds). The balanced reaction can be expressed as R-H + Cl-Cl --> R-Cl + H-Cl. So the bonds that are broken are C-H and Cl-Cl, the bonds made are C-Cl and H-Cl.... therefore heat of reaction = (105 + 58) - ( 84 + 103) = 163 - 187 = -24 kcal/mol.
e. The reaction is exothermic.
Common errors:
a. a few students did not balance oxygen correctly
b." fudged" calculations to get a positive heat of formation although their calculation was set up correctly - also the usual H2 heat of combustion being double in the calculation
c. confusion between larger number/more exothermic...also confusion between "boiling point" / heat of combustion
d. forgetting to include H-Cl in calculation, mixing up calculation to get an endothermic heat of combustion