Part 7: MECHANISMS
Note that no other reagents are needed in order to complete any
of these sequences, you should only be using what is there.
Common errors:
General:
i. Drawing curly arrows that were backwards... always electron rich to electron poor. ALWAYS! Or worse, not drawing arrows for some steps.
ii. Not balancing charges in each mechanistic step.
iii. For B or C not providing a justification
iv. Compressing several reactions steps in to one step.
v. Adding reagents not given in the question and therefore not needed and hence answering a different question!
Specific:
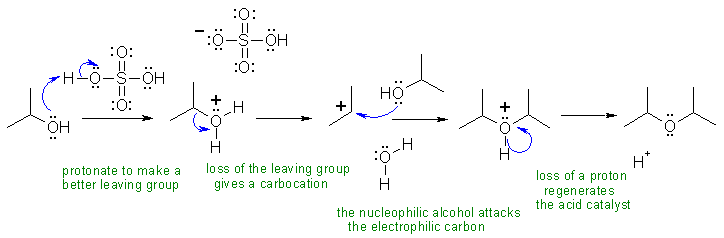
A: Generating the alkoxide ion (RO-) by using HSO4- or SO42- as a base in acid solution. Can't! Think about the pKas!
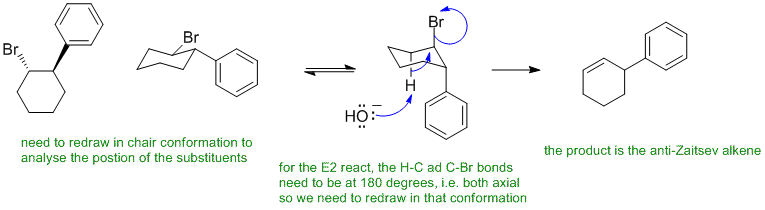
B: Ignoring the stereochemistry of the E2 pathway (i.e. H-C and C-LG bonds at 180 degrees), predicting the Zaitsev product, showing an E1 pathway via a carbocation, showing a substitution reaction despite the "classic" alkyl halide elimination reaction conditions (i.e. strong base / heat)
C: Missing the C+ rearrangement step, predicting the anti-Zaitsev product, showing an E2 type pathway, showing a substitution reaction despite the "classic" alcohol elimination reaction conditions.
A Alcohols can be both the electrophilic side and the nucleophilic side of substitution reactions. In this example, they are both ... this can be used for the synthesis of symmetrical ethers.

B Alkyl bromides undergo elimination via an E2 pathway when heated with a strong base (e.g. HO-). We need to consider the stereochemistry of the process:
C Alcohols undergo elimination via an E1 pathyway when heated with a strong acid:
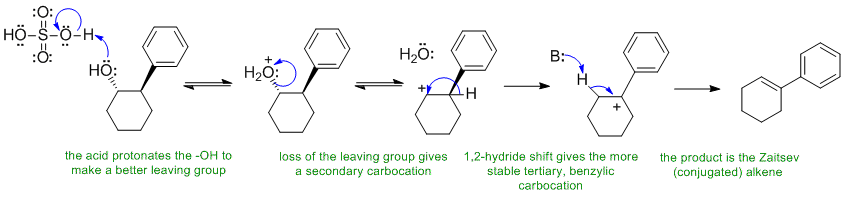
In this scheme, the base B: could be HSO4-, H2O or the alcohol itself.