a. The elemental analysis data gives the empirical formula to be C5H10O.
b. Given the MW = 86 g/mol, then since C5H10O = (5 x 12)+(10 x 1)+(1 x 16) = 86, then the molecular formula = C5H10O.
c. Index of hydrogen deficiencies ( IHD) = 1 (this helps us know that the structures must contain 1 unit of unsaturation based on double bonds or rings).
d. The IR band at 1715 cm-1 implies a C=O, and in order to have 5 C types and 4 H types, the C=O can't have an H so it has to be a ketone...
![]()
e.
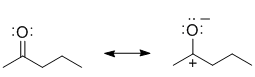
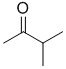
f. The IR band at 1715 cm-1 implies a C=O, and in order to have 4 C types and 3 H types, the C=O can't have an H so it has to be a different ketone...
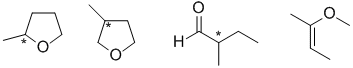
g. Stereocenters could mean chirality centers (C with 4 different groups attached) or alkenes that can be cis or trans, for example:
h. Since the IHD =1, if there are no sp2 atoms, then there will have to be a ring. The IR means no C=O an no OH, so to incorporate an O, it will have to be an ether.
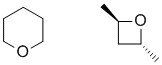
a. Not being able to do elemental analysis calculations (high school material) so use the link above to review.
c. Calculation errors
d & f. Counting types of H or C and putting it into practice.
e. Poor understanding of resonance, breaking the rules for resonance structures by moving atoms to create enol type structures.
g. Not drawing 4 different groups attached to the proposed chirality center.
h. Ignoring the IHD and hence not drawing an isomer of C5H10O.