Part
6: THERMODYNAMICS
This should have been a reasonably straight forward calculation, but care
needs to be taken to get the right structures and the right molecular formula,
then do the math correctly.
a. Cyclohexane and trans-hex-3-ene are constitutional isomers (since they have different connectivities, e.g. different functional groups).
b. C6H12 + 9 O2 --> 6 CO2 + 6 H2O
c. 
d.
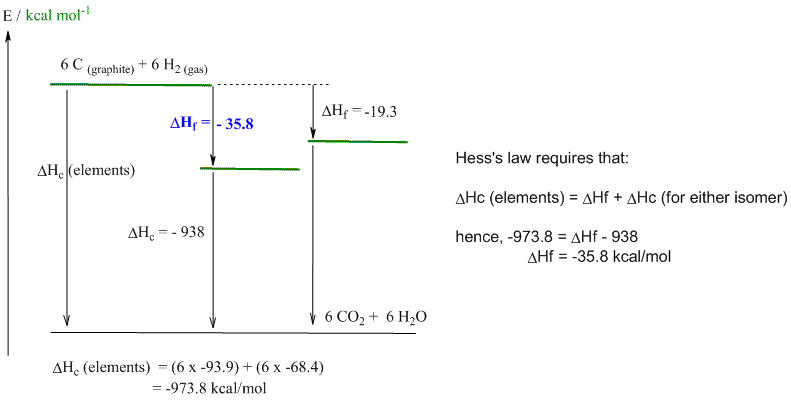
e. Cyclohexane is more stable (DHf = -35.8 kcal/mol) than trans-hex-3-ene (DHf = -19.3 kcal/mol) because it contains 6 sp3-sp3 C-C sigma bonds. In contrast hex-3-ene contains 5 C-C s bonds and 1 C-C p bond. p bonds are weaker than s bonds and hence an all CC s bond system will be more stable. C-C s bond = 88 kcal/mol, C=C = 146 kcal/mol, hence the p bond = 146 - 88 = 58 kcal/mol.
Common errors:
In part a :
- some students used two different terms to describe the relationship between the two isomers, only 1 term is required / appropriate since it is a specific relationship between the specified pair of structures. i.e. trans-hex-3-ene is a constitutional isomer of cyclohexane but it is a stereoisomer or geometric isomer of cis-hex-3-ene (which the question did not ask).
In part b :
- did not show the right products from the combustion reaction (i.e. CO2 and H2O)
- did not balance the coefficients
- wrong molecular formula for cyclohexane
In part d :
- Incorrect math, e.g. using 12 H2 instead of 6.
- getting the signs wrong by not remembering to apply the fact that making bonds releases energy (-ve) and breaking bonds requires energy (+ve)
In part e, lots of students made the following mistakes in thinking.... :
- the alkene to be more stable than the cycloalkane because the double bond is stronger than the single bond, but this is the wrong comparison since you need to compare the weaker p bond to the s bond.
- the cycloalkane was more stable because it was more branched (but it's less branched since it has less methyl groups!)
- cyclohexane has resonance (likely they were confusing it with benzene : ( )
- related stability to surface area or compactness... which have nothing to do with stability (they are factors in intermolecular forces for physical properties).
- that a more +ve (or less -ve) DHf was more stable.... not true, the more exothermic (i.e. more -ve) DHf, the more stable the compound.
![]()
