Part 7: MECHANISMS
Note that no other reagents are needed in order to complete any
of these sequences, you should only be using what is there.
Common errors:
General:
i. Drawing curly arrows that were backwards... always electron rich to electron poor. ALWAYS! Or worse, not drawing arrows for some steps.
ii. Not balancing charges in each mechanistic step.
iii. Not providing justifications for the questions that specifically asked for them.
iv. Compressing several reactions steps in to one step and therefore omitting / ignoring important intermediates.
v. Adding reagents not given in the question and therefore not needed and hence answering a different question!
Specific:
A: Missed the rearrangement, incorrect stereochemistry.
B: Deprotonation of the wrong H. Not counting C atoms.
C: Ignoring the stereochemistry of the E2 pathway (i.e. H-C and C-LG bonds needs to be at 180 or 0 degrees), predicting the Zaitsev product, showing an E1 pathway via a carbocation, showing a substitution reaction despite the "classic" alkyl halide elimination reaction conditions (i.e. strong base / heat).
D: Incorrect alkene product, invoking stereochemical issues as if the reaction is E2, missing the C+ rearrangement step, showing an E2 type pathway, showing incorrect alkene stereochemistry.
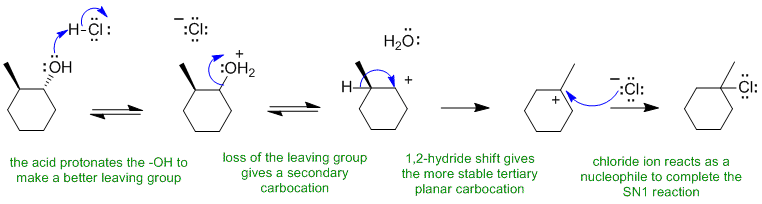
A
Alcohols react with HCl to give alkyl halides via SN1 pathways... need to make the O atom into a better leaving group by protonating before the nucleophile attacks or the leaving group leaves. The secondary carbocation that forms then rearranges via a 1,2-hydride shift to a more stable tertiary carbocation which then reacts with the chloride nucleophile to give the tertiary product.

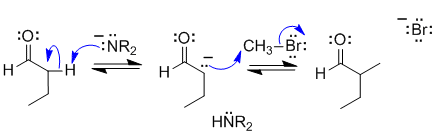
B
The aldehyde will be deprotonated by the amide ion in the strong base (LDA) in the alpha-position (adjacent to the carbonyl group) to form a nucleophile which then undergoes and SN2 reaction with the methyl bromide to give the alkylated aldehyde as the product.
C
Alkyl halides typically undergo elimination via an E2 pathway when heated with a strong base. However, in this case, it would require putting a t-butyl group (which is large) in an unfavourable axial position. Therefore, the reaction is more likely to be E1.
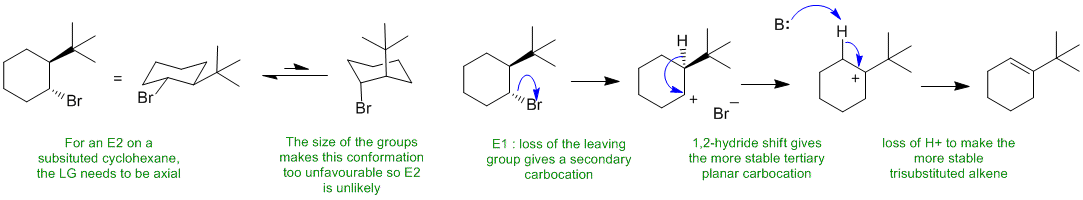
B: here would be the HO-
D
Alcohols undergo elimination via an E1 pathyway when heated with a strong acid. The change in the skeleton of the product compared to the starting material suggests a carbocation rearrangement has occurred.
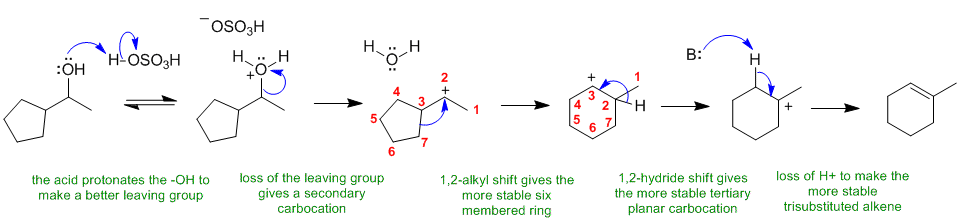
In this scheme, the base B: could be HSO4-, H2O or the alcohol itself