Part
5: STRUCTURE DETERMINATION
A
i. For a hydrocarbon, the formula for the index of hydrogen deficiency, IHD = 0.5 ( 2c+2-h), therefore, the IHD = 0.5 (2 x 6 + 2 -12) = 1
FYI : The IHD = pi + r (pi bonds plus rings)
there can be 1 units of unsaturation, which could mean a ring or a double bond.
ii.
![]()
iii. The IR data 1650 cm-1 implies an alkene, the 3100 cm-1 implies an sp2 C-H, i.e. alkene. To have only 3 C and H types, the C=C needs to be in the middle of the structure:
![]()
B
i. Possible functional groups based on two O and IHD = 1 : carboxylic acid, ester, ketone, aldehyde, alcohol, ether, alkene.
ii & iii pKa = 5 implies a carboxylic acid,
pKa = 15 implies an alcohol, and 20 suggests a ketone (or maybe an aldehyde).
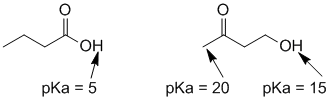
iv. Looking for a base that will deprotonate a carboxylic acid but not an alcohol, so ideally the looking for the conjugate acid with a pKa near 10. If it's near 5, then it will not really be strong enough to deprotonate the acid and if it's near 15 (e.g. HO- or RO-), then it will also deprotonate the alcohol. If pKa > 15 then it is too strong and will certainly deprotonate the alcohol. Good choices with pKa near 10 would be ammonia (NH3), phenolate (ArO-), thiolate (RS-), carbonate (CO32- ) or bicarbonate (HCO3-).
C
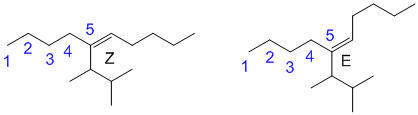
i. Longest chain is dec so C10. -ene indicates an alkene between C5-C6. There is a complex substituent also at C5. This substituent is a propyl, C3, chain with a methyl group at C1 and C2. The determine the stereochemistry, the complex substituent outranks the C4 chain at one end while the C4 chain outranks the H at the other. Z indicates the priority groups are on the same side, E that they are on opposite sides.
ii. Principle functional group is the -OH, an alcohol, so it needs to be named as such, -ol. Given that we have a 5 membered ring we have a cyclopentane system where the -OH needs to be at C1. The two alkyl groups are ethyl and methyl which need to be listed alphabetically. To determine numbering, starting with the -OH as 1, there is no first point of difference, so the numbering is determined based on the alphabetisation of the substituents. Hence 2-ethyl-5-methylcyclopentanol.
Common errors:
A.
Incorrectly calculating the IHD.
Incorrectly counting H and or C types.
Drawing structures for X that did not fit the molecular formula of C6H12 and the IHD.
Note that IHD defines the number of rings + pi bonds present for a given molecular formulae. The answer to part 1 should have helped you avoid this!
B.
Including an anhydride in the list of possible functional groups (it requires 3 O atoms).
Drawing structures for Y that did not fit the molecular formula of C4H8O2.
Drawing functional groups with incorrect pKa.
Suggesting bases that were too strong that would deprotonate both isomers of Y.
Suggesting the conjugate acid rather than the base. Think about what the pKa refers to.
C.
Incorrect E/Z isomers drawn, incorrect complex substituents, drawing that did not show the shape of the alkene unit correctly thus making the stereochemistry ambiguous.
Incorrect numbering, incorrect alphabetisation, missing that it was cyclic, wrong root name.