Part
6: THERMODYNAMICS
a. But-1-yne, buta-1,2-diene and buta-1,3-diene are constitutional isomers. (1 mark)
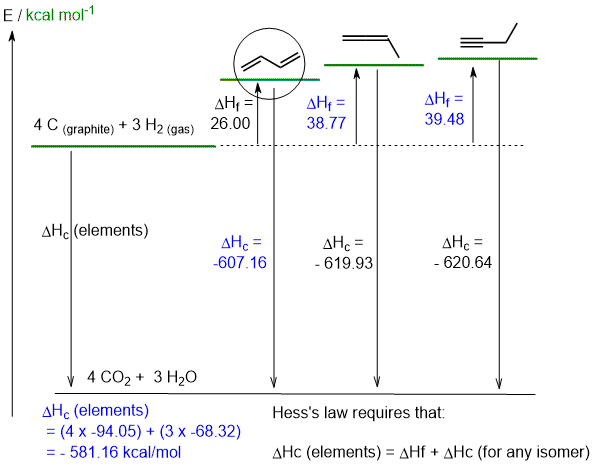
b. H2C=C=CHCH3+ 5.5 O2 -----> 4 CO2 + 3 H2O (2 marks)
c calculations are shown on the diagram (4 marks) and d. see below (4 marks)

e. 1,2-butadiene is H2C=C=CHCH3 and 1,3-butadiene is H2C=CHCH=CH2. Therefore 1,3-butadiene has 6 sp2C-H compared to 3 sp2C-H and 3 sp3C-H in the 1,2-isomer. There is also a pi interaction (resonance) between the C2 and C3 in 1,3-butadiene that further stablises the system. (2 marks)
Common errors: