Part 7: MECHANISMS
Note that no other reagents are needed in order to complete any
of these sequences, you should only be using what is there.
A i

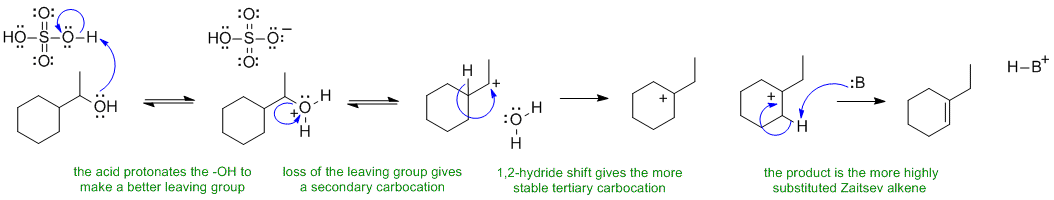
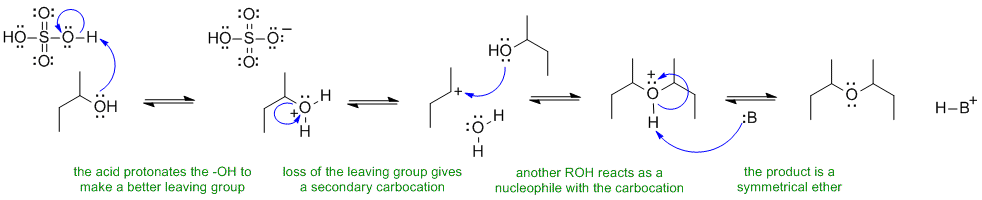
In the last step, the base :B could be the original alcohol, a water molecule or the conjugate base of sulfuric acid.
A ii The reaction that forms di-sec-butyl ether from 2-butanol is a substitution reaction. The reagent, sulfuric acid helps favour substitution over elimination (only some of the alcohol is protonated at any one tome so there is always ROH present to act as the nucleophile. The mechanism requires that the alcohol O be protonated first to make a better leaving group (-ve O groups are poor leaving groups).
The sulfate or bisulfate ion is a very poor nucleophile as the -ve charge is resonance stabilised. The final step is just an acid / base reaction to remove the proton. In the last step, the base :B could be the original alcohol, a water molecule or the conjugate base of sulfuric acid.
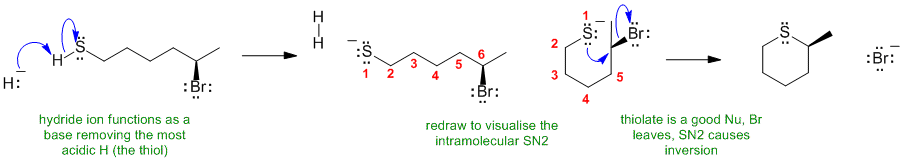
B i The acidic thiol is deprotonated by the hydride ion acting as a base to form a very good nucleophile which then undergoes an intramolecular SN2:
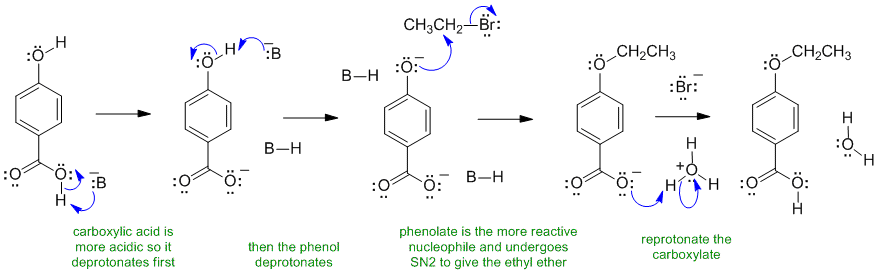
B ii The base here is the carbonate ion CO32-. It is shown as B- for simplicity. The more acidic carboxylic acid reacts first, then the phenol. Then the more nucleophilic phenol undergoes an SN2 reaction with the alkyl bromide to make the ether.
Common errors:
General:
i. Drawing curly arrows that were backwards... always electron rich to electron poor. ALWAYS! Or worse, not drawing arrows for some steps.
ii. Not balancing charges in each mechanistic step.
iii. Not providing justifications for the questions that asked for them.
iv. Compressing several reactions steps in to one step and therefore omitting / ignoring important intermediates.
v. Adding reagents not given in the question and therefore not needed and hence answering a different question!