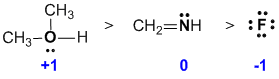| Qu1: |
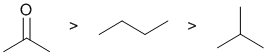
Boiling point is a physical property and is therefore controlled by intermolecular forces. The system with the strongest intermolecular forces will have the highest boiling point. The ketone has an electronegative atom which means there is a polar bond and a molecular dipole which means there are dipole-dipole interactions and a higher boiling point. Alkanes lack dipoles and therefore the intermolecular forces are that much weaker. If we look at the two isomeric alkanes, they have different branching. More branching makes the alkane more spherical and decreases the surface areas in contact - this means less intermolecular forces and therefore a low boiling point. (v1= B, v2 = AB) |
 |
| Qu 2: |
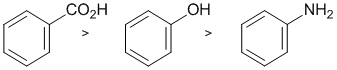
This question relates to the first laboratory expt of the semester and to acid/base chemistry. Carboxylic acids will react with sodium bicarbonate to deprotonate to make the more polar carboxylate, RCO2- which is water soluble. Alcohols tend to be more soluble than amines as the functional group is more polar due to the electronegativity of the heteroatom. (v1= A, v2 = B) |
 |
| Qu 3: |
Assign the formal charges...oxygen with 3 bonds and 1 lone pair is +1, nitrogen with 3 bonds is 0, and fluorine with 4 lone pairs is -1. (v1= C, v2 = A) |
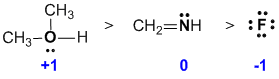 |
Qu 4: |
Basicity...Think about the availability of the electrons in the bases, always a good idea to draw in the lone pairs because lone pairs are more available than bonded pairs. Amines are more basic than alcohols ( O more electronegative than N => O is poorer e donor). In an alkane, all the e are involved in bonds (i.e. no lone pairs). (v1= D, v2 = C) |
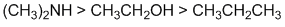 |
| Qu 5: |
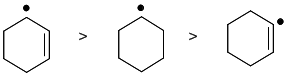
Radical stability order. Radicals are stabilised by alkyl groups and resonance, so secondary allylic are the more stable than simple secondary with the vinyl radical being the least stable. Vinyl radicals are less stable than alkyl radicals due to the low energy of the orbital that contains the single electron (less stabilisation). Alternatively, the most stable radical will be obtained from breaking the weakest bond (2o, allylic) and the least stable radical from breaking the strongest bond (vinyl sp2).(v1= E, v2 = B) |
 |
| Qu 6: |
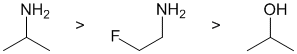
This is a question about basicity. (since we are looking at protonation). Think about the availability of the electrons in the bases, always a good idea to draw in the lone pairs because lone pairs are more available than bonded pairs. Amines are more basic than alcohols ( O more electronegative than N => O is poorer e donor). The nearby electronegative F will reduce the basicity of the amine group due to inductive effects. (v1= D, v2 = B) |
 |
| Qu 7: |
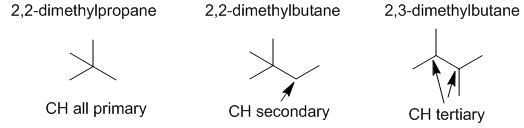
Radical stability order dictates the rate of the reaction with more stable radicals forming the fastest. Radicals are stabilised by alkyl groups so tertiary are the more stable than secondary than primary. (v1= AB, v2 = A) |
 |
| Qu 8: |
Counting types of H (models lab)? (v1= D, v2 = AB) |
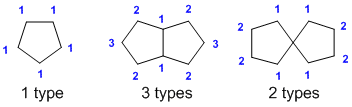 |
MOLECULAR PROPERTIES:
No real method here, really just do you know various aspects of molecular structure and apply it to the molecule(s) in question. |
| Qu9: |
The structure contains 3 rings, 9 C=C, 1 C=O and 1 CN (nitrile, triple bond = 2 units) so the index of hydrogen defficienc6y = 3 + 9 + 1 + 2 = 15. (v1 D, v2 C) |
| Qu10: |
Oxidation states...count the bonds attached to the atom being considered. A bond to a more electronegative atoms counts -1, a bond to the same type of atom counts 0 and a bond to a less electronegative atom (e.g. H) counts +1. Total the count and then consider the formal charge on the central atom since the oxidation state for the central atom plus the groups attached must equal the atoms formal charge. For C2 the C is attached to 3 x C (count 0), 1 x Cl (count -1) therefore total = -1 and therefore the oxidation state C2 = +1. (v1= AB, v2 = D) |
| Qu11: |
Functional groups....it is an amide (v1 = C, v2 = D) |
| Qu12: |
N9 is part of a triple bond in the nitrile and has two attached groups (C and lone pair) so the lone pair is in an sp hybrid orbital. N11 is part of a double bonds with three attached groups (2 C and lone pair) and is sp2 hybridised so the lone pair is in an sp2 hybrid orbital. (v1= D, v2 = A) |
| Qu13: |
N17 is adjacent to a C=O and is part of an amide where the N lone pair is in resonance with the C=O and therefore in order to allow the N lone pair to interact with the pi system, N17 is sp2 hybridised.
N23 is
simple amine, the N has 3 C atoms attached plus the lone pair, so N23 is sp3. (v1 = B, v2 = D) |
| Qu14: |
C22 is attached to 1 other C and is therefore primary, and is adjacent to a C=C so it is allylic (v1 = AD, v2 = AC) |
| Qu15: |
The strongest bond will be the triple bond in the nitrile. (v1 = B, v2 = D) |
| Qu16: |
For C20-C21 we have C=C and need to assign the E/Z stereochemistry, using the Cahn-Ingold-Prelog rules to assign group priorities....C > H and C > H at each end of the double bond with the higher priority C atoms on opposite sides = E (v1 = A, v2 = D) |
| Qu17: |
The N atoms in amines are the most basic (have lone pair, less electronegative, smaller atom) (v1 = AB, v2 = AD) |
SPECTROSCOPY:
Use the IR spectra provided to get the functional groups that are present in the structures, so look for the key groups : C=O (near 1700 cm-1) , -OH or -NH (above 3000 cm-1), aromatic C=C (two bands 1600-1400 cm-1), C-O (near 1200 cm-1) and triple bonds (near 2200 cm-1).... use the tables as needed. |
| Qu18: |
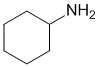
The IR does not show a C=O near 1700 cm-1. The IR does shows the double absorption at about 3300 cm-1 for -NH2. On closer inspection, the C-H stretches are < 3000 cm-1 indicating sp3 C-H only. This would be consistent with the primary amine (v1 = AE, v2 = D) |
 |
| Qu19: |
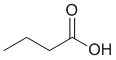
The IR shows a C=O near 1700 cm-1 and a strong and broad OH stretch at 2500-3500 cm-1. On closer inspection, the C-H stretches are < 3000 cm-1 indicating sp3 C-H only. This would be consistent with an aliphatic carboxylic acid. (v1 = B, v2 = AC) |
 |
| Qu20: |
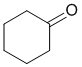
The IR shows a C=O a little above 1700 cm-1. The IR does not show -OH or -NH, or C=C, or triple bonds. On closer inspection, the C-H stretches are < 3000 cm-1 indicating sp3 C-H only. This would be consistent with the ketone. (v1 = E, v2 = BC) |
 |
| Qu21: |
The IR does not show a C=O near 1700 cm-1. On closer inspection, the C-H stretches are above and below 3000 cm-1 indicating sp2 and sp3 C-H suggesting the presence of an alkene (weak C=C at 1650 cm-1) (v1 = D, v2 = AE) |
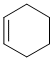 |
| Qu22: |
The IR does not show a C=O near 1700 cm-1. On closer inspection, the C-H stretches are are < 3000 cm-1 indicating sp3 C-H only. This would be consistent with a simple alkane (v1 = C, v2 = AD) |
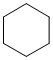 |
| Qu23: |
The IR shows a C=O just below 1700 cm-1 The IR does not show -OH or -NH, but there looks to be C=C near 1600 cm-1. On closer inspection, the C-H stretches are above and below 3000 cm-1 indicating sp2 and sp3 C-H suggesting the presence of an aromatic. This would be consistent with an aromatic ketone. (v1 = AB, v2 = A) |
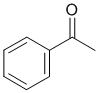 |
NOMENCLATURE:
Two types of questions here, however it is a good idea to use key descriptors like E and Z, R or S, cis or trans, numbering, functional group etc. to help screen the answers before getting too tied up in the words. For the first four, name the compound and then match it to the list, for the last four
use the key descriptors and check each molecule in turn. So this section really depends on the nomenclature rules. |
| Qu24: |
An alkane with the longest chain C9, so a nonane. Keeping the substituents simple, there are three substituents, a simple ethyl group and two methyl groups. The first point of difference dictates using 3,4,7 over 3,6,7 and alphabetisation means that the dimethyl group is named after the ethyl group since the multiplier is ignored when alphabetising such systems. Hence, 4-ethyl-3,7-dimethylnonane. (v1 = E, v2 = E) |
|
| Qu25: |
The longest chain containing the C=C is C7, so a heptene. Number to give the alkene the lowest number means hept-3-ene. At C3 there is a methyl group and at C4 there is an amino group. The double bond is E (priority groups on opposite sides) so we have (E)-4-amino-3-methylhept-3-ene. (v1 = D, v2 = A) |
|
| Qu26: |
The principle functional group is the alkene and those two C atoms need to be 1 and 2. It's a six membered ring (cyclohexene). Then the first point of difference rule requires that the system be numbered as a with the chloro group at C1 and since the alkene has to involve C1 and C2, then the bromo is at C6. Alphabetisation means that the bromo group needs to be before the chloro group. This means we have 6-bromo-1-chlorocyclohexene. (v1 = D, v2 = AB) |
|
| Qu27: |
The longest chain is C6 with an alkene and a ketone so hexenone. The ketone is the principal functional group which needs to be numbered as 3 (lowest) which makes the alkene C4 i.e. hex-4-en-3-one. The alkene is trans. Hence we have trans hex-4-en-3-one. (v1 =B, v2 = E) |
|
| Qu28: |
All of the systems are ethers, it's all about the common alkyl substituents.
isobutyl is -CH2CH(CH3)2 and isopropyl is -CH(CH3)2. (v1 = B, v2 = E) |
|
| Qu29: |
Substituted benzenes and an ester. The methyl group forms the alcohol part of the ester and the ethyl group is on the benzene. ortho means the two groups are 1,2- to each other. (v1 =D, v2 = C) |
|
| Qu30: |
R/S stereochemistry
Chirality center is marked with a *. Priority of groups there is OH > C=C > C > H. With OH forwards, the chirality is R, so we need the OH to be back. (v1 = D, v2 = C) |
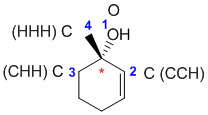 |
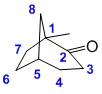
| Qu31: |
The bicyclic system has 8C and a ketone so it is an octanone. The [3.2.1] indicates the number of C is each link between the bridgehead carbons. Need to start to number from a bridgehead, going along the longer link first to give the ketone the lowest number and hence locate the methyl group. (v1 = E, v2 = D) |
 |