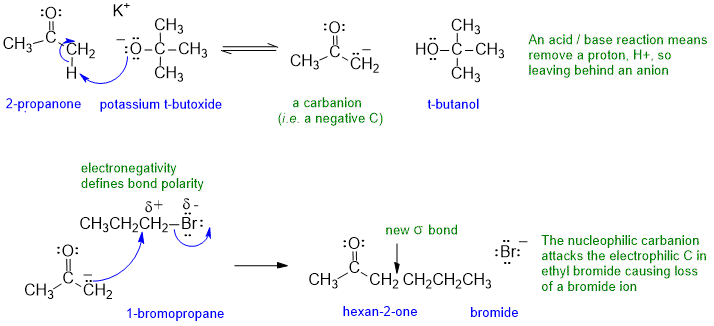
The following diagram shows the solution to the mechanistic question. Note that all the information applies to a single reaction sequence that has been completely described verbally. There is no need for extra reagents or extra steps etc. The curly arrows are drawn specifically to match the text in the question. The biggest problem students have is making sure they understand the language of chemistry. Most students have trouble because they can't draw the structures from the IUPAC names. Read the words carefully, and then make the curly arrows tell that same story. Remember curly arrows go from electron rich to poor and to balance the charges at each step.

If you struggled with this part of the question, first draw the compounds whose names were provided, then think about the types of reactions (e.g. acid / base) and try to fill in the structures in the gaps, then finally add the required curly arrows to account for all the bonding changes.
In this problem, if you didn't know what potassium t-butoxide was, then you should have been able to work it out from the knowing what t-butanol was and that t-butoxide forms t-butanol after an acid - base reaction (i.e. simple proton transfer) and given that it tells you that t-butoxide is a base in the question....
The significant resonance structures can be drawn by breaking the C=O and distributing the charges in accord with electronegativity. Though minor contributors, these are potentially important. For the carbanion, there is contributor has a C=C and negative O.
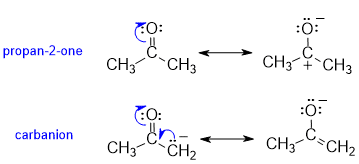
Acidity : Propanone is more acidic than propane as indicated by the lower pKa (20 versus 50+ respectively). This is because the conjugate base of propanone is resonance stabilised (see the carbanion above) where the negative charge on C can be delocalised to the more favourable (i.e. more stable) location on the electronegative O atom. Propane, only has a simple, unstabilised carbanion.
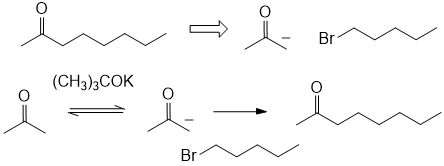
If you wanted to prepare octan-2-one using this type of reaction, you should react the carbanion of propanone with 1-bromopentane) instead of the 1-bromopropane (as stated in the question the alkylating agent adds an alkyl group) that was used in the first part of the question (count C atoms... in the question 3 + 2 gives 5.... need 8 have 3.... use C5 alkylating agent).
Common errors:
Mechanism:
1. Incorrect structure of named compounds (count the C atoms).
2. Drawing the conjugate base as a carbocation (+ve) and not a carbanion as indicated (and as it is in reality).
3. Incomplete curly arrows (need to account for all bonding changes)
4.Trying to draw radical reactions or drawing arrows that were not electron rich to electron poor.
Resonance:
1. Drawing structures with different overall charges.
2. Drawing structures that were NOT resonance structures (e.g. that implied atoms had moved)
3. Showed equilibrium arrows instead of the double headed resonance arrow.
Acidity:
1. Vague answers that felt like recitals of memorised facts rather than true application.
2. Mentioning only either resoance or the electronegativity of O but not both
3. Only talking about the functional group and not a "why"
4. Stating that is was because of a lower pKa (which is not a rationale of why and thus does not answer the question).
Synthesis:
1. Failed to apply the facts in the question to a new scenario.
2. Gave incomplete answers by missing out reagents.
3. Did not count C and do the math!