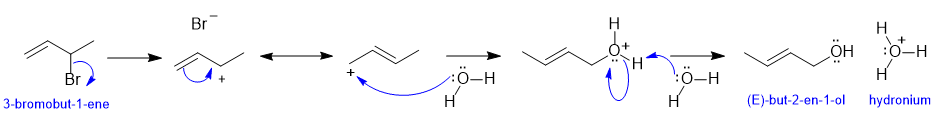
If you struggled with this part of the question, first draw the compounds whose names were provided, then think about the types of reactions (e.g. acid / base) and try to fill in the structures in the gaps, then finally add the required curly arrows to account for all the bonding changes.
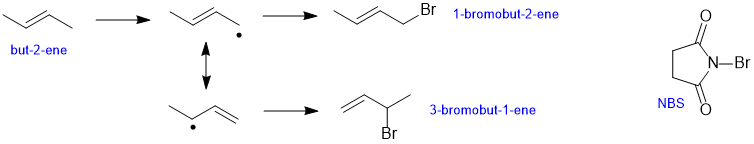
b. But-2-ene will undergo radical subsitution (as stated in the question) with NBS via an allylic radical. That allylic radical has two different resonance contributors. Each contributor leads to a different product, see the outline of the scheme below: (2.5 marks)
c. Draw the required product (an ether) and then compare to part a and make use of a similar strategy: (2.5 marks)
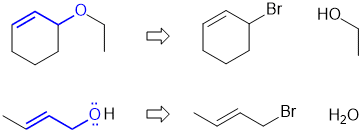
Common errors:
Adding extra steps not in the question, using radical arrows instead of double headed arrows (as the question specifically stated was required), drawing carbanions instead of carbocations, incorrect electrons counts (carbocations are not radicals and don't have an unpaired electron).
a. i. Curly arrows backwards (always e- rich to poor), omitting or incorrect formal charges. ii incorrect use / misuse of the resonance arrow iii. Attacking the wrong carbocation iv. going from a C4 carbocation to a C3 system when the oxygen attacked v. not checking the name of the final product.
b. Products need to be stable molecules, they are not radicals or carbocations etc. There were lots of answers about different types of H and primary, secondary and tertiary postions that didn't relate to the specifics of the question (lack of thought about the answer). Almost everyone wrote words instead of potentially drawing a simple scheme... Many answers talked about either HBr or succinimide being the other product, but those are not good answers....
c. did not apply the reaction in part a to help solve part c, could not draw the target molecule (required product) correctly based on the name provided (it's an ether)..