Part 8: THERMODYNAMICS
This should have been a reasonably straight forward calculation, but care needs to be taken to get the right structures and the right molecular formula, then do the math correctly.
a. C5H10 + 15/2 O2 --> 5 CO2 + 5 H2O (2 marks)
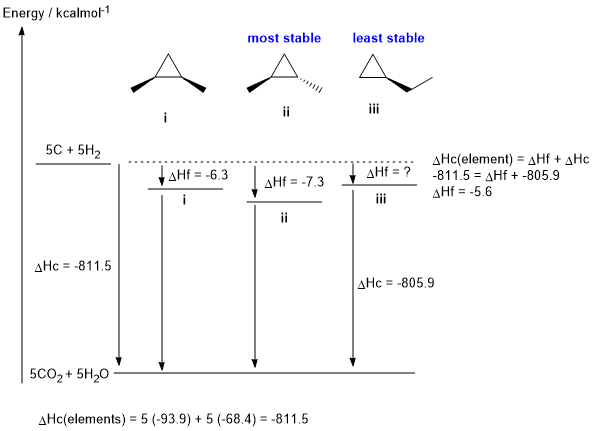
b. The calculation is shown on the figure below. (3 marks)
c. The values are matched on the figure below. (2 marks)
d.(3 marks)

e. Stability will be affected by the degree of branching (more stronger primary C-H bonds will cause a more stable molecule) and the steric interactions between the groups (from the molecular models experiment). Based on branching, isomers i and ii are more stable than isomer iii since they have one more branch. The difference between i and ii is due to the cis and trans nature of the methyl groups. In isomer i where the methyl groups are cis, the two methyl groups are held in the unfavourable eclipsed position which destablises isomer i with respect to isomer ii. (2 marks)
f. (1 mark)
![]()
Common errors:
In part a:
In part d :