Part 7: MECHANISMS
Note that no other reagents are needed in order to complete any
of these sequences, you should only be using what is there.
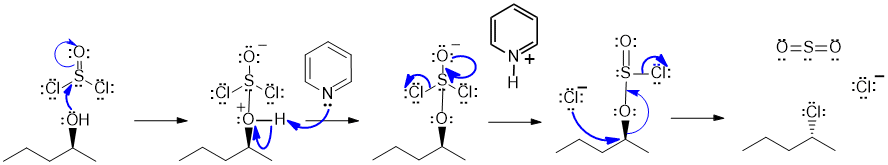
A i Reaction of an alcohol with thionyl chloride:

Since the stereochemistry of the product at the -OH group has been inverted (i.e. wedge to hash), we need to show an SN2 mechanism.
A ii Acid catalysed dehydration of an alcohol:
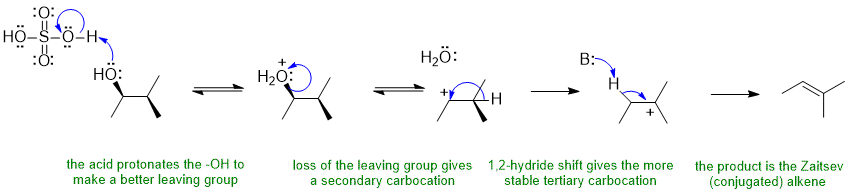
Alcohols undergo elimination via an E1 pathyway when heated with a strong acid. The change in the skeleton of the product compared to the starting material suggests a carbocation rearrangement has occurred. In this scheme, the base B: could be the -HSO4, ROH or H2O each of which is present in the reaction mixture.
B i
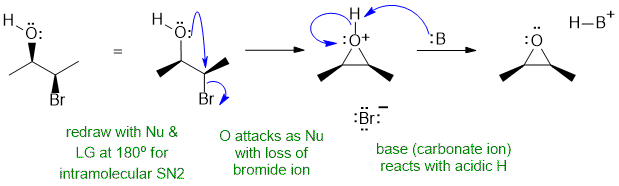
An intramolecular reaction of an alcohol reacting with a alkyl bromide to give a cyclic ether (an epoxide). Need to address the stereochemistry of the reaction to get the correct stereochemistry of the epoxide product. Here the base B: is the carbonate ion, CO
B ii
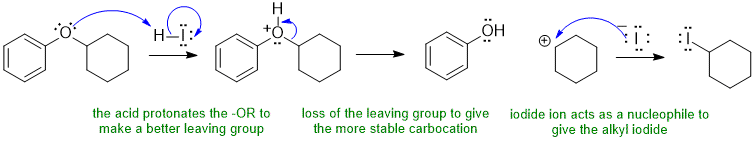
Ethers are closely related to alcohols. Alcohols react with HI (a strong acid) to form alkyl iodides typically via an SN1 pathway with protonation of the -OH to make a better leaving group, C+ formation and then reaction of the nucleophilic bromide ion with the electrophilic C+... the ether will do the same kind of thing. Make sure to make the more stable C+.
Common errors:
General:
i. Drawing curly arrows that were backwards... always electron rich to electron poor. ALWAYS! Or worse, not drawing arrows for some steps.
ii. Not balancing charges in each mechanistic step.
iii. Compressing several reactions steps in to one step and therefore omitting / ignoring important intermediates.