Part 6: THERMODYNAMICS
This should have been a reasonably straight forward calculation, but care needs to be taken to get the right structures and the right molecular formula, then do the math correctly.
a. C6H10 + 17/2 O2 --> 6 CO2 + 5 H2O (1 mark)
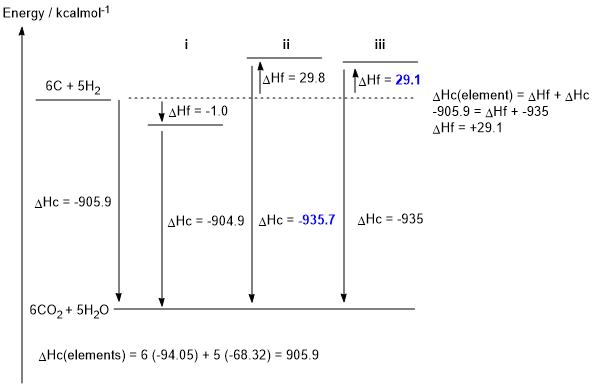
b. The caculation is shown on the figure below (2 marks, 1 each)
c. (4 marks)

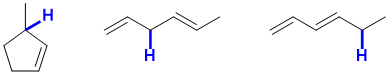
d. Possible examples of weaker C-H would include a tertiary allylic C-H, or a doubly allylic system. For possible examples, see below: (2 marks)
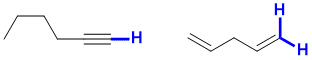
e. Possible examples of stronger C-H would include a sp C-H in a terminal alkyne or a primary vinyl C-H. For possible examples, see below: (2 marks)
f. If there are 3 different monochlorination products, the starting material requires 3 different types of sp3 C-H. Two possible answers are shown below: (2 marks)

Common errors: