Note that a phenol is not typically soluble in 5% NaHCO3 solution.
Part 5: STRUCTURE DETERMINATION
Note that there is no point showing all possible answers here, so the most likely and potentially simplest answers are shown.
a. C8H8O2 has a molecular weight (8 x 12.01) + (8 x 1.008) + (2 x 16.00) = 136.14 g/mol (1 mark)
b. The formula for index of hydrogen deficiency, IHD = 0.5 ( 2c+2-h), therefore, the IHD = 0.5 (2 x 8 + 2 - 8) = 5 (1.5 marks)
(note that an IHD of 5 is large for a small MF, so it might suggest a benzene ring as part of the structure)
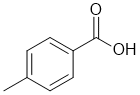
c. To be soluble in 5% NaHCO3 solution (solubility expt) it suggests a carboxylic acid :

Note that a phenol is not typically soluble in
5% NaHCO3 solution.
d. IR of 3300cm-1 suggests an -OH and soluble in NaOH solution but not NaHCO3 solution suggests a phenol but not a carboxylic acid (3 marks)
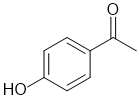
e. pKa > 20 suggests some sort of an ester with alpha-H or a terminal alkyne but can't save any -OH (too acidic) (2 marks)
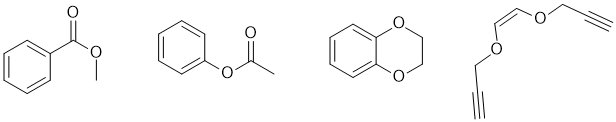
f. No peak between 1685-1800 cm-1 implies no C=O (2 marks)
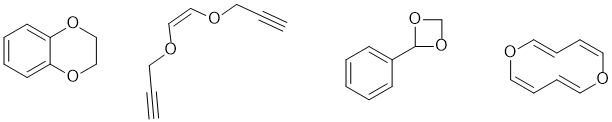
g. Oxygen containing functional groups that could be present in X with up to 2 x O atom and a unit of unsaturation could include : carboxylic acid, ester, aldehyde, ketone, alcohol, ether, epoxide, enol (1.5 marks)
Common errors: