Part 6: THERMODYNAMICS
This should have been a reasonably straight forward calculation, but care needs to be taken to get the right structures and the right molecular formula, then do the math correctly.
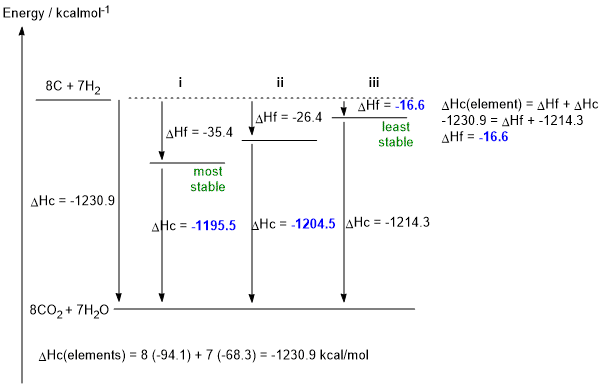
a. C8H14 + 11.5 O2 --> 8 CO2 + 7 H2O (1 mark)
b. DHf iii = -1230.9 - (- 1214.3) = -16.6 kcal/mol (2 marks)
c. DHc i = -1230.9 - (- 35.4) = -1195.5 kcal/mol (1 mark)
DHc ii = -1230.9 - (- 26.4) = -1204.5 kcal/mol (1 mark)
d. (5 marks)

e. Isomer iii is the only chiral molecule. Neither i nor ii are chiral because both have at least one plane of symmetry OR because neither have an sp3 C atom with 4 different groups attached(2 marks)
f. A bicyclo[2.2.1] system requires a two ring system where there are 2 "bridges" with 2 C atoms and 1 bridge with 1 C atom (1 mark)
![bicyclo[2.2.1]](351mt21th_f.gif)
Common errors: