Part 7: MECHANISMS
Note that no other reagents are needed in order to complete any
of these sequences, you should only be using what is there.
A

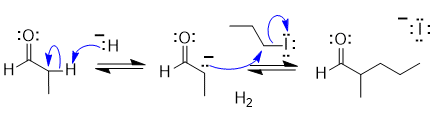
The aldehyde will be deprotonated by the hydride ion (a strong base) in the alpha-position (adjacent to the carbonyl group) to form a carbanion nucleophile which then undergoes and SN2 reaction with the propyl bromide to give the alkylated aldehyde as the product.
B
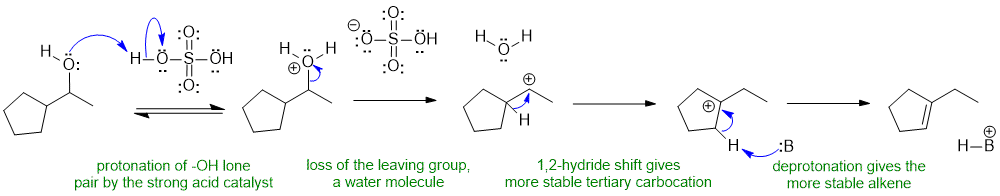
Alcohols react with sulfuirc acid to give alkenes via E1 pathways... need to make the O atom into a better leaving group by protonating before the nucleophile attacks or the leaving group leaves. The secondary carbocation that forms then rearranges via a 1,2-hydride shift to a more stable tertiary carbocation which then is deprotoanted by a base to give the Zaitsev product. In this case, the base, B:, could be the conjugate base of the acid, HSO3-, the alcohol, or a water molecule.
C
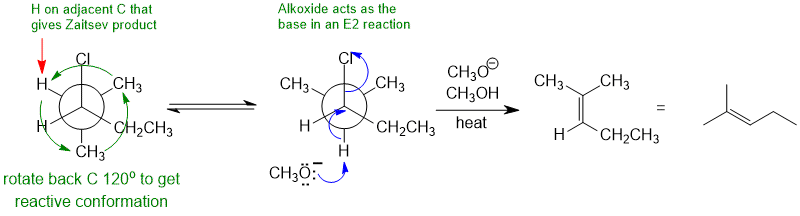
If an alkyl chloride is heated with strong base (here it's methoxide), then a 1,2-elimination occurs to give an alkene. Alkyl halide eliminations are typically E2. This prefers an anti arrangement (180 degrees) of the C-H and C-LG bonds (this needs to be addressed by redrawing the Newman projection with the H-C and C-Cl being set up anti. In this reaction, the base is methoxide (i.e. small), so the Zaistev elimination is going to dominate - this tells us which H will be removed by the base.
Common errors:
General:
i. Drawing curly arrows that were backwards... always electron rich to electron poor. ALWAYS! Or worse, not drawing arrows for some steps.
ii. Not balancing charges in each mechanistic step.
iii. Compressing several reactions steps in to one step and therefore omitting / ignoring important intermediates.