Part 8: SPECTROSCOPY
The following data is available from the
question.
Note
: Remember to cross
reference things to confirm.... e.g.
IR may show C=C, use NMR to
confirm that etc.
MS: M+
seen at 175 g/mol, no m, m+2 pattern so that indicates no Cl or Br. The odd MW tells us an odd number of N are present.
IR:
There is none of the really strong absorptions : no C=O, OH, NH. There are absorptions of note at about 2250 (C≡C or C≡N) and 1610 & 1505 cm-1 which are probably Ar C=C. Given the possibility of an alkyne, there is no sp C-H at 3300 cm-1
13C NMR:
The normal proton decoupled spectrum shows a total of 9 peaks indicating 9 types of
C. By analysis of the chemical shifts, we have 5 peaks between 110-160 ppm (unusual) and peaks at 56 & 3 between 35 and 15 ppm that are
most likely from sp3 C hydrocarbon fragements.
1H NMR:
The proton spectrum shows a total of 6 sets of peaks indicating 6 types of H.
|
d/ppm
|
multiplicity
|
integration
|
Inference
|
|
6.95
|
d
|
2
|
ArH 2 x CH coupled to H |
| 6.85 |
d
|
2
|
ArH 2 x CH coupled to H |
|
3.8
|
s
|
3
|
-CH3 not coupled, deshielded |
| 2.7 |
t |
2 |
-CH2 coupled to 2H |
| 2.45 |
t |
2 |
-CH2 coupled to 2H |
| 1.95 |
p |
2 |
-CH2 coupled to 4H |
(q = quartet, t = triplet, d = doublet, s = singlet)
The most significant structural information
from this are:
- adding integrals shows 13H (or a multiple thereof)
- it has a disubstituted aromatic (benzene)
- there are coupling patterns to decipher
Summary....
The MS indicated MW = 175 and N.
The IR showed the presence of a triple bond.
13C NMR suggests a benzene system with 9 types of C
H NMR gives -CH2-, -CH2-, -CH2-, -CH3, and C6H4 (disubs. benzene)
This information suggests an initial molecular formula = C10H13N
which gives MW = (10x12) + (13x1) + 14 = 147 which means we are missing 28
The missing 28 can be accounted for by another C (note that the C-NMR suggests this due to the 5 peaks above 110 ppm) and an O (H-NMR peak at 3.8ppm suggests a possible -OR group)
Therefore, molecular formula = C11H13NO which has an IHD = 6 consistent with the triple bond and benzene.
Altogether...
|
The fragments we have are:
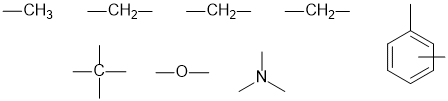
The IR, 13C and molecular formula imply that the triple bond is a nitrile, C≡N.
The H-NMR 3H singlet at 3.8 ppm indicates an -OCH3.
The two 2H triplets at 2.7 and 2.45 ppm and the 2H pentet at 1.95 ppm indicate a group, -CH2-CH2CH2- group.
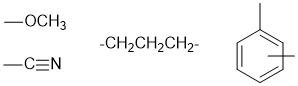
Given the H-NMR shifts of the end -CH2- groups of the alkyl chain (both below 3ppm), we can deduce that that chain is connect to the benzene and the nitrile group and therefore that the methoxy group is a substituent on the benzene ring.
All that is left to do is establish the regiochemistry of the Ar region which is most easily done from the NMR. 4 types of ArC and 2 types of ArH as "doublets" implies a para- system, giving us the structure... |
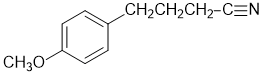
4-(p-methoxyphenyl)butanenitrile
|
The final step should always be to check what you
have drawn. The easiest thing to check is usually the coupling patterns you
would expect to see, and the chemical shifts of each unit. You should
be asking yourself : "Does my answer give me what the H-NMR shows ?"
Common errors:
MS incorrectly read the MW for the identified M+ (check the scale), missed the N.
IR missed the triple bond.
H NMR had hydrocarbon pieces that did not fit the integration and coupling patterns.
General
- did not check MW with data from MS
- did not check HNMR integration for number of H on the aromatic and hence had incorrect substitution of the benzene.
![[Home]](../mol.gif)


![[Home]](../mol.gif)