Part 7: MECHANISMS
Note that no other reagents are needed in order to complete any
of these sequences, you should only be using what is there.
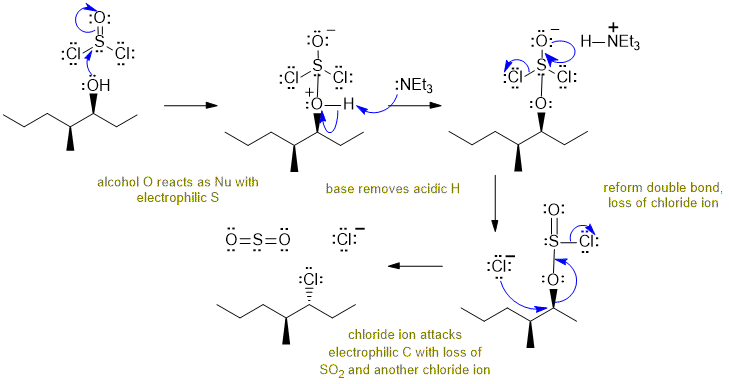
A Reaction of an alcohol with thionyl chloride:

Alcohols react with thionyl chloride / base to give alkyl chlorides via an SN2 pathway. Commons errors specific to this question : incorrect structure of thionyl chloride, showing SN1 not SN2, not managing the stereochemistry, not showing the formation of SO2.
B Reaction of an alcohol with a hydrogen halide:
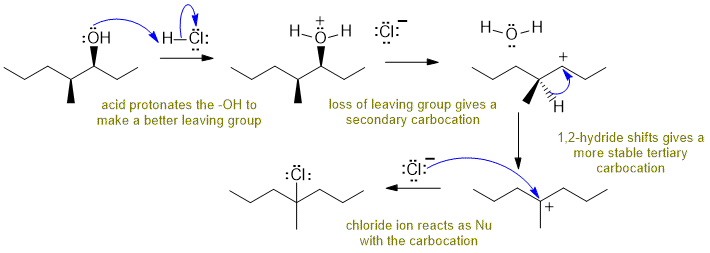
Alcohols react with HCl to give alkyl chlorides via SN1 pathways... need to make the O atom into a better leaving group by protonating before the either the nucleophile attacks or the leaving group leaves. The secondary carbocation that forms then rearranges via a 1,2-hydride shift to a more stable tertiary carbocation which then is attacked by the chloride ion nucleophile. Commons errors specific to this question : missed the hydride shift, incorrect arrows for the hydride shift, arrows for the protonation / deprotonation of the OH.
C
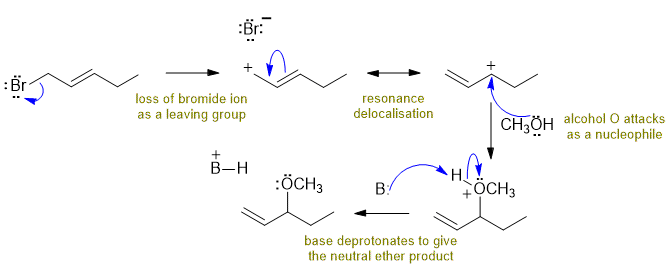
The allylic bromide loses the bromide ion to give a resonance stabilised carbocation which is then attacked by the methanol ultimately giving an methoxy ether. In this question, the base would be the sodium carbonate. Commons errors specific to this question : managing the pKa of carbonate compared to methanol (it's not a strong enough base to deprotonate a simple alcohol).
D
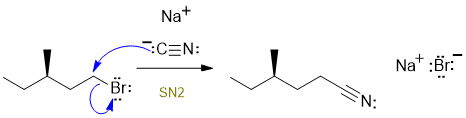
A primary alkyl halide with NaCN in DMF (a polar aprotic solvent) = SN2. Commons errors specific to this question : incorrect management of stereochemistry (it only affects the center where the bonds are made and broken which is not a chirality center in this question), incorrect or incomplete structure of the cyanide ion, showed SN1 (primary C+ ???) not SN2.
Common errors:
General:
i. Drawing curly arrows that were backwards... always electron rich to electron poor. ALWAYS! Or worse, not drawing arrows for some steps.
ii. Not balancing charges in each mechanistic step.
iii. Compressing several reactions steps in to one step and therefore omitting / ignoring important intermediates.