Part 6: MECHANISM
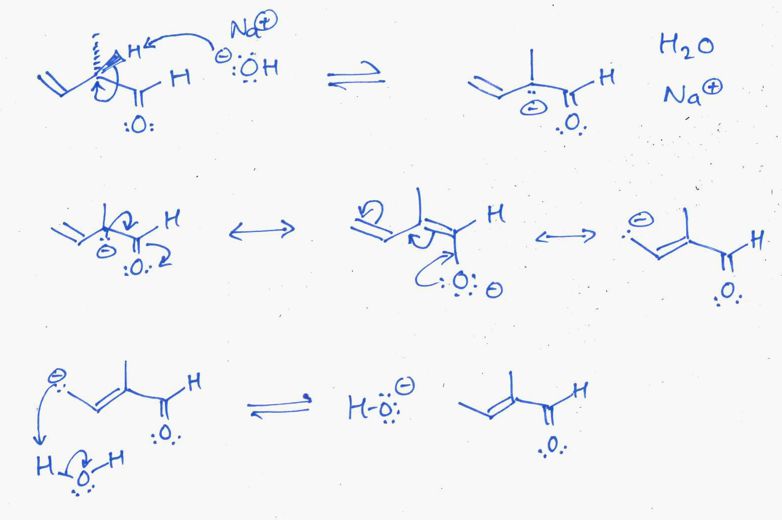
The following diagram shows the solution to the mechanistic question. Note that all the information applies to a single reaction sequence that has been completely described verbally. There is no need for extra reagents or extra steps etc. The curly arrows are drawn specifically to match the text in the question. The biggest problem students have is making sure they understand the language of chemistry. Most students have trouble because they can't draw the structures from the IUPAC names (that means they don't know their nomenclature well enough). Read the words carefully, and then make the curly arrows tell that same story. There is NO need for extra steps. Remember curly arrows go from electron rich to poor and to balance the formal charges at each step - errors on formal charges were common.

The product contains the required C=O (an aldehyde) and the C=C is more stable because it is (1) more substituted tri- vs mono- and (2) because it is part of a conjugated (extended) pi system.
b. The likely stereoisomers of the product are shown below. If naming as cis and trans, then the term is defined by the shape of the root name (i.e. in this case, the shape of the but-2-enal unit. If the methyl and the aldehyde are on the same side the alkene is cis, if they are on opposite sides, then the alkene is trans. If naming as E or Z, then one needs to use the Cahn-Ingold-Prelog priority rules that looks at the atomic number at the first point of difference. For the methyl vs the hydrogen, the C > H, for the methyl vs the aldehyde, the C(OOH) > C(HHH) because O > H (atomic number).

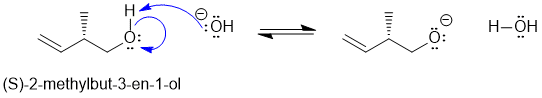
c. No.... in this case, the most acidic H is now on the O atom of the alcohol. Given that the acid / base properties of ROH and H2O are similar, they have similar pKas. Therefore, hydroxide, HO- can react as a base to generate an equilibrium amount of the conjugate base, the alkoxide. However, since the negative charge is on the O atom, attached to an sp3 C, the negative charge can not participate in resonance and therefore you can't convert this system to a more stable isomer using the analogous reaction.