Part 5: STRUCTURE DETERMINATION
Note that there is no point showing all possible answers here, so the most likely and potentially simplest answers are shown.
a. C5H11NO has a molecular weight (5 x 12.01) + (11 x 1.008) + (1 x 14.01) + (1 x 16.00) = 101.15 g/mol (1 mark)
b. The formula for index of hydrogen deficiency, IHD = 0.5 ( 2c+2-h+n), therefore, the IHD = 0.5 (2 x 5 + 2 - 11 + 1) = 1 (1 mark)
c. Nitrogen functional groups that could be present : amide, amine (imine, oxime, nitroso) (1 mark )
d. No sp2 atoms means no pi bond, so the IHD = 1 (above) will require a cyclic system. 3 types of C and H requires some symmetry. (3 marks)

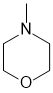
e. IR near 1650 cm-1 tends to suggest a C=O and to have a pKa = 30 would suggest a tertiary amide (the acidic H are those on the C adjacent to the carbonyl group, indicated by the arrow) (2 marks)
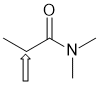
f. A pKa = 15 could suggest a primary amide or an alcohol. PKa = 15 would suggest amide NH (indicated by arrow), so either a primary amide (shown) or a secondary amide. (2 marks)
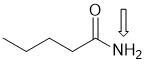
g. A tri-substitued alkoxy (-OR) alkene (C=C) that has Z stereochemistry (3 marks)
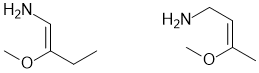
Common errors: