Part 6: THERMODYNAMICS
a. Structures i and ii are constitutional isomers. (0.5 marks)
b. Structures i and iii are constitutional isomers. (0.5 marks)
c. 5-methylhex-1-ene (1 mark)
d. C7H14 + 10.5 O2 --> 7 CO2 + 7 H2O (1 mark)
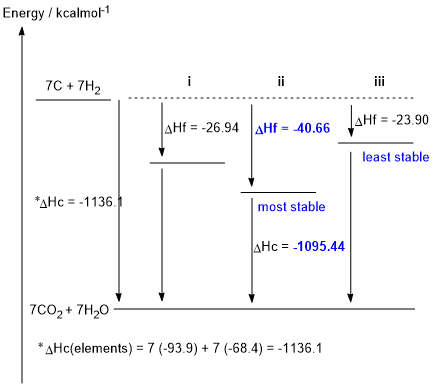
e. DHc elements = (7 x -93.9) + (7 x -68.4) = -1136.1 kcal/mol
DHf ii= -1136.1 - (- 1095.44) = -40.66 kcal/mol (1 mark)
f & g

g.Given the thermodynamic data in the table provided in the question, and as shown in the figure above, ii is the most stable isomer (i.e. lowest energy) and iii is the least stable isomer (1 mark)
h. ii, the most stable isomer, is a cycloalkane. The primary contribution to its stability is that ii has all the CC bonds are sigma bonds while i and iii each contain a double bond and hence a pi bond. CC sigma bonds are stronger than pi bonds. For i versus iii, the issues are increased branching or the degree of substitution of the alkene unit increases the stability of the system. Increased branching has the net effect of increasing the number of stronger bonds, for example, the number of primary C atoms and hence the number of primary C-H bonds. Since, in terms of bond strength, primary C-H bonds > secondary C-H > tertiary C-H, a molecule with more primary C-H (more branching) is more stable (1.5 marks)
Common errors: