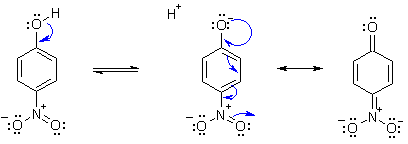
Part 6: MECHANISM
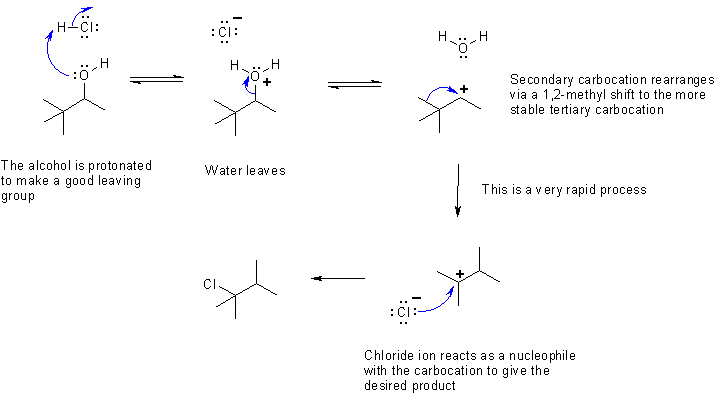
a) The mechanism is a substitution reaction of an alcohol with HCl to give a re-arranged chloroalkane (that is the new substituent is not attached to the same carbon skeleton as the starting material). The fact that the carbon skeleton rearranges indicates that a carbocation was formed, and therefore, that the process is SN1 rather than SN2.
The key parts are protonating the -OH to make a good leaving group (-OH will not act as a leaving group to give hydroxide), loss of the leaving group to give the secondary carbocation, rapid rearrangement to the more stable tertiary carbocation and attack by the chloride nucleophile. An SN2 reaction would have given the un-rearranged product, 2-chloro-3,3-dimethylbutane.
The mechanism is shown below:

b) The nitro group is an example of an
electron withdrawing group by
RESONANCE.
When such a group is in the ortho- and / or para-
positions
they can stabilise the phenolate anion (see below) by delocalising the
-ve charge onto the O atom of the nitro group. With more nitro groups,
there is more delocalisation and hence greater stablity of the anion,
making
it easier to form and thus lowering the pKa of the parent acid.
IT IS VERY IMPORTANT to note that the same RESONANCE effect is NOT possible with a nitro group in the meta position. Try drawing the curly arrows to prove this to yourself. This is reflected by the higher pKa compared to the para- nitrophenol. The change in pKa compared to phenol itself is due to the inductive effect of the +ve N of the nitro group.