Part 8: STRUCTURE DETERMINATION
The question is based on the Lucas test
of a series of isomeric alcohols.
The Lucas test is an SN1 type of reaction where the alcohol is
converted
into a chloroalkane. This occurs by protonating the -OH to make the
better
leaving group, H2O. The isomeric chloroalkanes with their
names
are shown below.
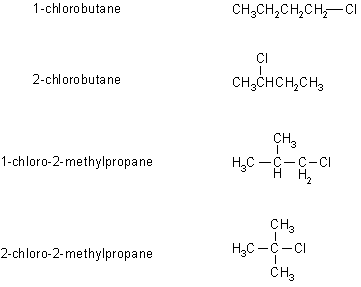
A is the 2-chloro-2-methylpropane (t-butylchloride) since it is produced in a fast reaction and the tertiary carbocation is the most easily formed. The H-nmr of A would be a single peak, so it must be I.
B is 2-chlorobutane since it is produced via a moderately fast reaction which would indicate a secondary carbocation. The H-nmr of B would have four sets of peaks, since there are four types of H, integrating 1:2:3:3 and would be a quintet, a quartet, a doublet and a triplet as shown in III.
C and D are both formed
in slow reactions and a therefore
primary systems. The H-nmr of 1-chlorobutane has a characteristic
deshielded
triplet (as in IV) and 1-chloro-2-methylpropane a deshielded
doublet
(as in II). Based on the data provided, it is not possible to
say
which is C or D specifically.