From the combustion analysis data we get 85.53% C and 14.37% H, and we are told that the MW = 70.14 g/mol.
This can be used to give the empirical
formula as CH2 (HOW
?)
and the hence the molecular formula = C5H10 (check
the MW !)
This gives the IHD = 1 by using : 0.5 x ( 2 x 5 + 2 -10), telling us we can have either a double bond OR a ring.....
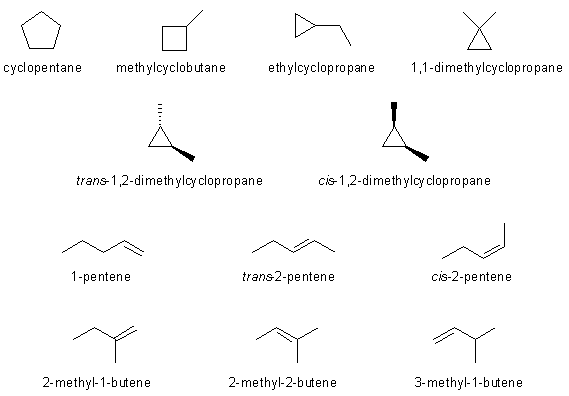
Here are the possible isomers and their names:

Constitutional (also known as structural) isomers are compounds with the same molecular formula, but with different branching patterns or different functional groups. From the compounds above, any pair other than those that are configurational isomers (see below) are constitutional isomers.
Configurational isomers are compounds with the same molecular formula that differ ONLY by the spatial arrangement of the groups. This means they contain the same bonds and the same functional groups. The only pairs of configurational isomers here are either cis- and trans-2-pentene or cis- and trans-1,2-dimethylcyclopropane.
The most stable alkene with be the most highly substituted alkene, which is 2-methyl-2-butene and the most stable alkane (which has to be cyclic), will be the one with the least ring strain, cyclopentane.