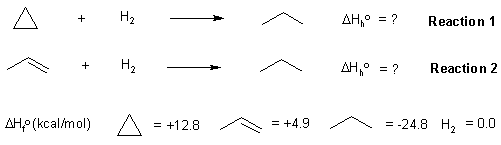
Here is a diagram showing the reactions and the data provided in the question:
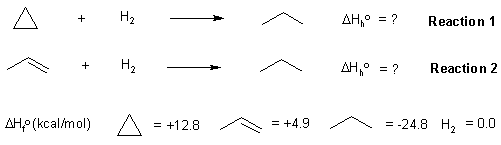
The heat of a reaction (in this case the heat of hydrogenation) can be calculated from the heats of formation of the products and starting materials (also see the figure below):
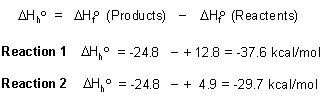
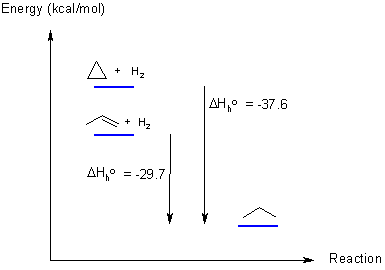
With this data, we can create the required energy diagram:
If a C=C is 146 kcal/mol and a C-C is 83
kcal/mol, then we can estimate
the strength of a p bond as 146 - 83 = 63 kcal/mol.
This means a s bond is 20 kcal/mol stronger
than a p bond.
We need to compare the reactions of
cyclopropane and propene to propane.
The reaction of cyclopropane breaks a s
bond while the reaction of propene a p bond.
Remember that breaking a bond requires an INPUT of energy and
therefore,
20 kcal/mol more energy was required to break the s
bond in cyclopropane compared to breaking the p
bond in propene. If we correct for this difference by thinking of
it in terms off making the s bond "weaker" to
make it more like the p bond, then the heat
of hydrogenation of cyclopropane becomes -57.6 kcal/mol. (i.e.
more
exothermic by 20 kcal/mol).
But the ring strain of cyclopropane will be RELEASED on going to
propane.
Now we can get an estimate of the energy
released due to ring strain
by -57.6 - - 29.7 = -27.9 kcal/mol
This compares very favourably with 28 kcal/mol found by combustion
analysis.