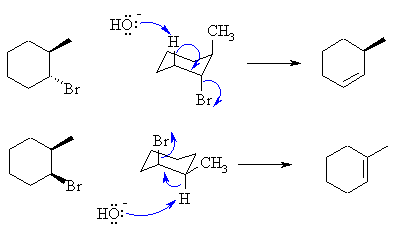
| These reactions are E2 reactions since we have a
secondary
bromide in the presence of a strong base. The E2 reaction is a
concerted
process that requires the H and LG to be at 180o
(antiperiplanar).
In cyclohexanes, this requires that the Br be in an axial position.
In the trans-isomer, elimination to the less stable alkene occurs since the Me group is also in the axial position. In the cis-isomer, the more stable alkene can be produced since the H is in the correct orientation. |
 |

![[Chem 351 Home]](../mol.gif)