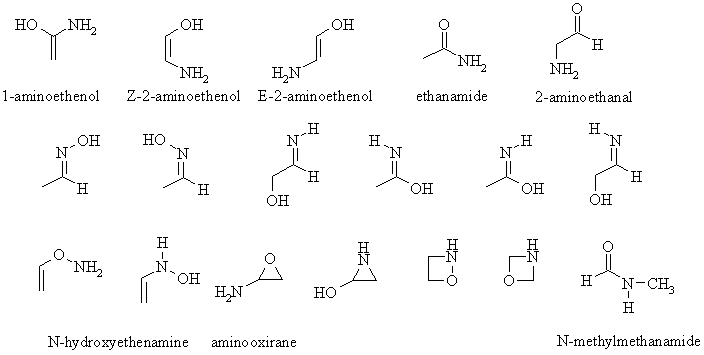
EA:
Since is does
not add up to 100%, we should assume the "lost" % is oxygen.The % data
allows one can get the empirical formula = C2H5NO.
By checking the MW of the empirical formula (2x12) + (5x1) + 14 + 16 =
59, we know that the molecular formula is also C2H5NO.
Here is a list of possible
isomers
:

The pairs of geometrical isomers are
adjacent to each other in the figure
and are named as E- or Z-.
Any other pair of compounds would be constitutional isomers.
Resonance.... Well only those structues with pi bonds can exhibit resonance. The forms shown above would be the major contributors and any with charge separation would be less important.