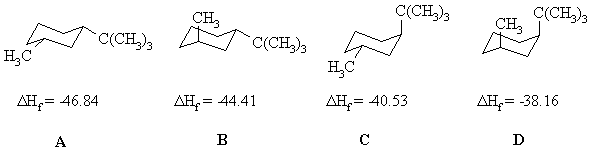
Here is a diagram showing the four isomeric 1-tert-butyl-3-methylcyclohexanes and the DHf data:

The ΔHf values are assigned on the basis of stability. The more stable the isomer, the more exothermic the ΔHf. (see diagram below if that troubles you). In order to determine the stability order, we need to look at the positions of the two substituents. Equatorial substitutents are prefered over axial substituents due to the presence of the destabilising 1,3-diaxial interactions (a steric effect) when the substituent is axial due to its proximity to the other axial positions on the same face of the cyclohgexane. The larger the substituent, the more destabilising this steric effect is. So the most stable isomer will be the one with both Me and tBu equatorial, then the one with the larger tBu equatorial and Me axial, then equatorial Me, axial tBu, and the both axial isomer will be the least stable.
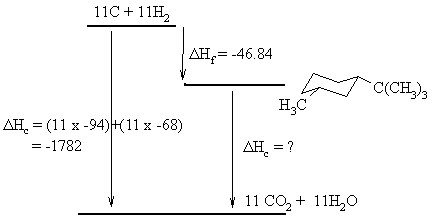
The ΔHc of the most stable isomer can be calculated using the Hess's Law relationship for the balanced reactions shown to the left. From the diagram we get the relationship that:
ΔHc (elements) = ΔHf (isomer) + ΔHc (isomer).
So, based on the molecular formula we
can insert the numbers to get
: -1782 = -46.84 + ΔHc, therefore ΔHc
= -1735.16 kcal/mol.
To calculate the equilibrium
constant, we need to use the equation ΔG
= -RT ln K.
In order to use this we need to approximate ΔG
= -46.84--38.16 = -8.68 kcal/mol (for the two cis isomers).
Thus inserting the values, -8680 cal/mol = -(1.987 cal/molK)
(298 K) ln K, so K = 2.3 x 106