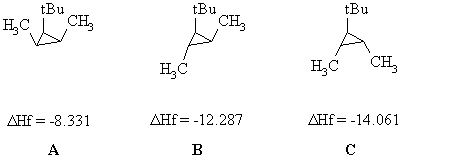
Here is a diagram showing the three stereoisomeric 1-tert-butyl-2,3-dimethylcyclopropanes and the DHf data:

The ΔHf values are assigned on the basis of stability. The more stable the isomer, the more exothermic the ΔHf. (see diagram below if that troubles you). In order to determine the stability order, we need to look at the relative positions of the three substituents. In A all the substituents are eclipsing each other and there is a large amount of steric interaction between the alkyl groups as they are all on the same side of the cyclopropane. The destabilises A and makes it the least stable. In B we have -Me and t-Bu eclipsing and their steric interaction. The interactions in C are reduced because the two smaller groups are the ones now close together so it is the most stable.
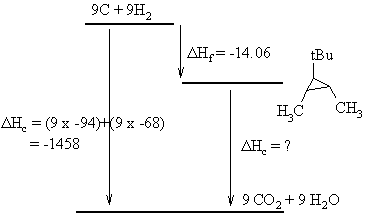
The ΔHc of the most stable
isomer
can be calculated using the Hess's Law relationship for the balanced
reactions shown to the left.
From the diagram we get the relationship that:
ΔHc (elements) = ΔHf (isomer) + ΔHc (isomer).
So, based on the molecular formula we
can insert the numbers to get
:
-1458 = -14.06 + ΔHc,
therefore ΔHc
= -1443.94 kcal/mol.
To calculate the equilibrium
constant, we need to use the equation
ΔG
= -RT ln K.
In order to use this we need to approximate ΔG
= -14.06--8.33 = -5.73 kcal/mol (for the A to C).
Thus inserting the values, -5730 cal/mol = -(1.987 cal/molK)
(298 K) ln K, so K = 16,000