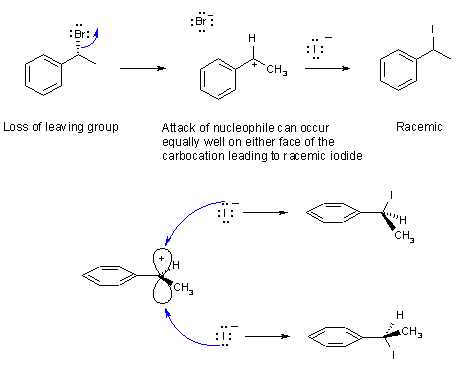
Part 6: MECHANISM
a) The mechanism is a substitution reaction of an (R)-bromoalkane with sodium iodide to a racemic iodoalkane The fact that racemisation occurs indicates that a resonance stabilised, planar carbocation was formed, and therefore, that the process is SN1 rather than SN2. An SN2 reaction would require that the stereochemistry was inverted.
The key parts are loss of the leaving group to give the secondary carbocation, and attack by the iodide nucleophile.
The mechanism is shown below:

b) This part is all about acidity and brings in several features...
Propane (pKa = 50) is not very acidic because the conjugate base is an unstablised carbanion. Remember that C is not very electronegative.
Ethanol (pKa = 15) is more acidic that propane because the conjugate base is an alkoxide (RO-) with the charge localised on an electronegative atom, oxygen.
Propanone (pKa = 19) is more acidic than propane because the carbanion has another resonance contributor that moves the negative charge to a more electronegative atom, the oxygen. This stabilises the conjugate base compared to that of propane.
Acetic acid (pKa = 5) is the most acidic
because it has two important
resonance structures, both of which have the negative charge on
electronegative
oxygen atoms.