Part 7: SPECTROSCOPY
This was not an easy problem. It is made
difficult by the lack of the
molecular formula (though it can be calculated) and the number of C / H
units which gives a lot of potential isomeric structures to consider.
The following data is available from the
question:
MS: M = 174 (Even, therefore
zero or an even number of N, no
isotope pattern for Cl or Br)
EA:
The % data
allows one can get the empirical formula = C4H7O2,
but when used together with the MW data available from the MS, the
molecular
formula = C8H14O4 . From here we get
the
IHD
= 2
IR:
There are significant
absorptions at 1735cm-1 due to a C=O and at
1150cm-1
which could be a C-O. It is also important to note the absence of bands
for -OH, (so not an alcohol or carboxylic acid) especially with the
amount
of O from the EA. calculations.
13C
nmr: The proton
decoupled spectrum shows only 4 peaks indicating 4 types of C. Since we
know the formula, we know there must be some symmetry. By analysis of
the
chemical shifts, we get that 173 ppm is typical of O=C-O unit, 61 ppm
is
C-O and those at 29 ppm and 17 ppm are most likely just from
hydrocarbon.
1H
nmr: First of
all we only have 3 types of H showing up. After this, it's a good idea
to tabluate the information to make sure you get it all correctly
matched
up:
|
d/ppm
|
multiplicity
|
integration
|
Inference
|
|
4.2
|
quartet
|
2
|
CH2 coupled to 3H, deshielded, probably by O |
|
2.7
|
singlet
|
2
|
isolated, uncoupled CH2 deshielded, probably by C=O |
|
1.3
|
triplet
|
3
|
CH3 coupled to 2H |
The simplest information we get from the
H nmr here is from the integrals
(total of 7H) which, when compared to the formula, tells us we have a
symmetrical
system. The most significant structural information in the H nmr data
is
that we have an ethyl group -CH2-CH3 . Since the
-CH2- is also deshielded, this suggests it is actually an
ethoxy
group -O-CH2-CH3.
So with all this information we have the
following pieces: 2 x -O-CH2-CH3,
2 x -CH2- and 2 x C=O. The IR frequency of the C=O and the
13C
nmr both suggest that we have an ester system. Once we have that then
it
falls together....
Altogther...
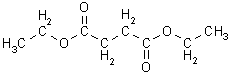 The
real "tough" part here is that although the 2 middle -CH2-
units
are attached to each other, there is no coupling between them because
they
are of the same chemical type, and hence they appear as a singlet. This
is the only solution that is consistent with all the data.
The
real "tough" part here is that although the 2 middle -CH2-
units
are attached to each other, there is no coupling between them because
they
are of the same chemical type, and hence they appear as a singlet. This
is the only solution that is consistent with all the data.
The final step should always be to check
what you have drawn. The easiest
thing to check is usually the coupling patterns you would expect to
see,
and the chemical shifts of each unit.
You should be asking yourself : "Does my answer give me what the
H-nmr shows ?"
 The
real "tough" part here is that although the 2 middle -CH2-
units
are attached to each other, there is no coupling between them because
they
are of the same chemical type, and hence they appear as a singlet. This
is the only solution that is consistent with all the data.
The
real "tough" part here is that although the 2 middle -CH2-
units
are attached to each other, there is no coupling between them because
they
are of the same chemical type, and hence they appear as a singlet. This
is the only solution that is consistent with all the data.