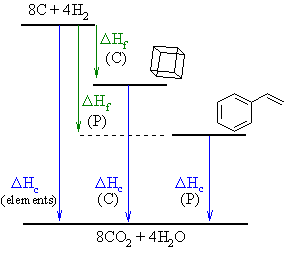
First note that both cubane (C) and
phenylethene (P) are C8H8
i.e. they are isomers.
It is easiest to solve the question by first sketching out the
information
(note that it doesn't matter if the relative postions of the cubane and
phenylethene are wrong, the math will still work).

Then, based on Hess's law we get
two expressions, one for cubane, one for phenylethene:
ΔHf (C) + ΔHc
(C) = DHc (elements), so ΔHf
(C) = -983.6 - -1365.5 = 381.9 kcal/mol
and
ΔHf (P) + ΔHc
(P) = ΔHc (elements), so ΔHc
(P) = -983.6 - 35.11 = -1018.7 kcal/mol
Note that the positive heats of formation just mean that the two compounds are unstable with respect to the elements and that the sketch should be redrawn, but that doesn't stop us from getting the correct answer to the problem.
So as cubane is less stable since it has a more exothermic heat of combustion and a more endothermic heat of formation than phenylethene it is at higher energy.
Yes it is reasonable to compare them because they are isomeric structures.
Cubane contains 6 cyclobutane rings that have to be planar, so there is plenty of ring strain due to angle strain and torsional strain due to the enforced planarity of the system. These factors destablise cubane. In constrast, phenylethene is a conjugated system which stabilises it.