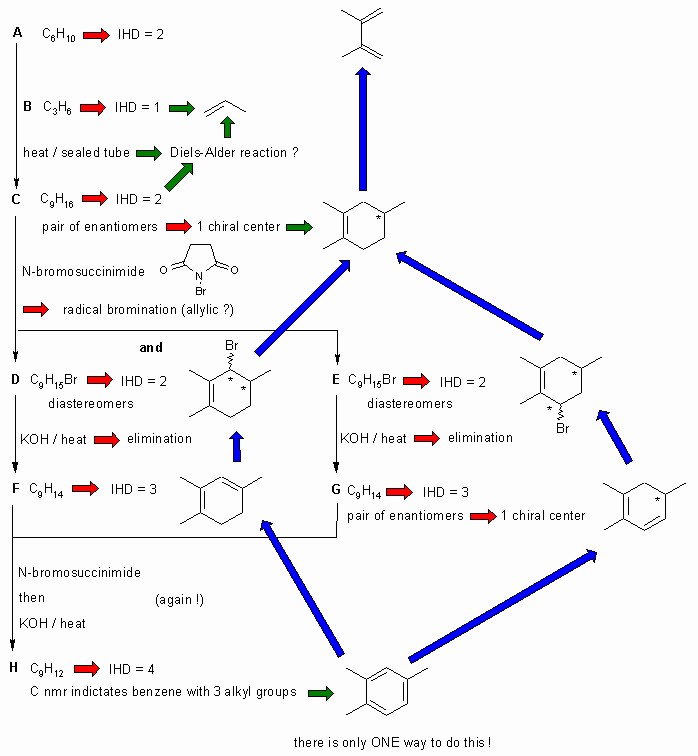
A schematic of the solution is shown below. The information from the question is given in black. Deductions directly from this information are given by red arrows. Points that provide potential key information are shown by green arrows which leads to the structures which are linked via the blue arrows to show the path required to work them all out. There are, of course, other possible thought pathways. The potential key steps are recognising the Diels-Alder reaction and the trimethylbenzene product H. Note that A can not be 1,4-dimethyl-1,3-butadiene as then C would contain 3 chiral centers.

A = 2,3-dimethyl-1,3-butadiene
B = propene
C = 1,2,4-trimethylcyclohexene
D = 3-bromo-1,2,4-trimethylcyclohexene
E = 6-bromo-1,2,4-trimethylcyclohexene
F = 1,2,4-trimethyl-1,3-cyclohexadiene
G = 1,2,5-trimethyl-1,3-cyclohexadiene
H = 1,2,4-trimethylbenzene