Part 6: MECHANISMS
Note that no other reagents are needed in order to complete
any of these sequences, you should only be using what is there.
A
Reaction of a ketone with an alcohol to form a hemi-ketal in an
intramolecular
sense. Protonate the ketone carbonyl to make it more reactive,
then
make the alcohol attack as a nucleophile.
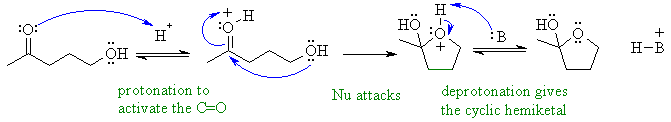
In this mechanism, B: could be C=O groups, ROH or the conjugate base of the acid catalyst.
B
Decarboxylation of a b-ketoacid under acidic
conditions. The loss of the CO2
gives the enol which then
undergoes acid catalysed tautomerisation to the ketone.
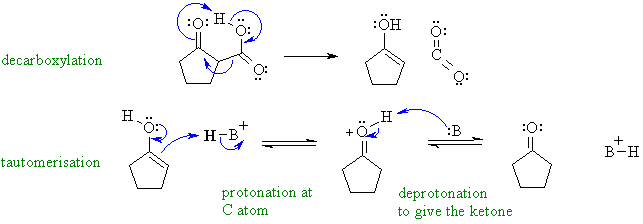
In this mechanism, B: could be C=O groups, -OH groups or the conjugate base of the acid catalyst.
C
Hydration of an alkyne gives the
Markovnikov product, which is the
more highly substituted enol via the more stable resonance stabilised
secondary
vinyl cation which then undergoes acid catalysed tautomerism to the
ketone.
Notice that the proton donor is the acid catalyst and the nucleophile
is
water (no hydroxide under acidic conditions).
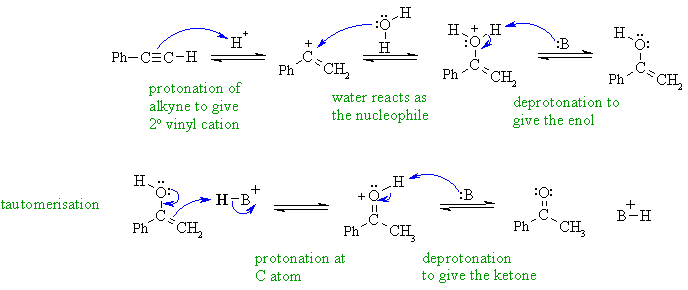
In this mechanism, B: could be C=O groups, -OH groups C=C groups, or the conjugate base of the acid catalyst.
D
A Friedel-Crafts type alkylaion
reaction. The acid protonates
the alcohol making a better leaving group. This leaves giving the
carbocation,
which is the electrophile that adds to the aromatic system, via the
arenium
ion which then loses a proton so restoring the important aromatic
character.
In the final step the base, :B could be the conjugate base of the
acid, a water molecule or the original alcohol.
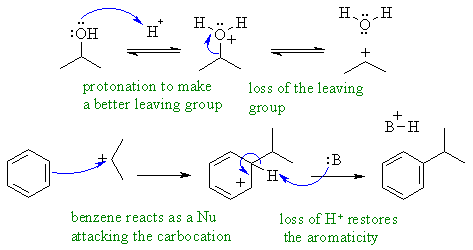
In this mechanism, B: could be ROH, H2O or the conjugate base of the acid catalyst.
![[Chem 353 Home]](../mol.gif) Return
to Homepage
Return
to Homepage




