Part 6: MECHANISMS
A
Halogens react with alkenes via an electrophilic addition mechanism,
via a cyclic halonium ion intermediate which is then opened by the
attack
of a nucleophilic species. In this case the electronegativity
difference
of Br and Cl makes the bromine the electrophile that adds to the alkene
and the chloride the nucleophile that attacks the halonium ion.
When
the chloride ion attacks, it attacks the more substituted end since the
cationic character of the intermediate leads to carbocation character
which
is most pronounced at the secondary end.

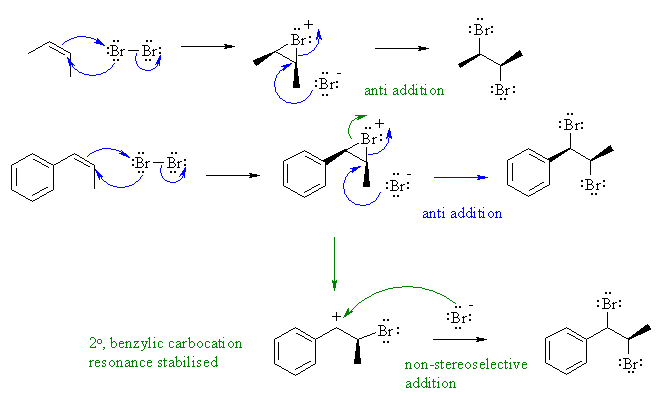
B
Bromine react with alkenes via an
electrophilic addition mechanism,
via a cyclic bromonium ion intermediate which is then opened by the
attack
of a nucleophilic species. This attack of the nucleophile normally
results
in an overall anti addition of the two bromine atoms as is seen here
with
2-butene.
However, Z-1-phenylpropene is not as
stereoselective. In this
case, the bromonium ion can open to give a very stable resonance
stabilised
carbocation. This planar carbocation could be attacked on either face
by
the bromide ion and so is not stereoselective. The 73% selectivity for
anti indicates that these two pathways are competing with each other.
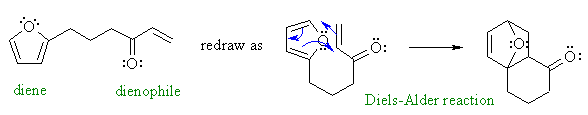
C
The bridged nature of the product and
the diene / dienophile portions
of the starting material, plus the note that no other reagents are needed
should suggest an intramolecular variation of a Diels-Alder reaction:
![[Chem 353 Home]](../mol.gif) Return
to Chem 351/3 Homepage
Return
to Chem 351/3 Homepage



