Part 6: MECHANISMS
Note that no other reagents are needed in order to complete
any of these sequences, you should only be using what is there.
A
HBr in the presence of peroxides will be a radical addition type
process
with a radical chain type process. This means it needs to be drawn with
single headed arrows indicating the motion of individual electrons.
Note
that the question indicates that you should be considering an excess of
HBr. The major product turns out to be the 1,2-dibromide for reasons
explained
below.
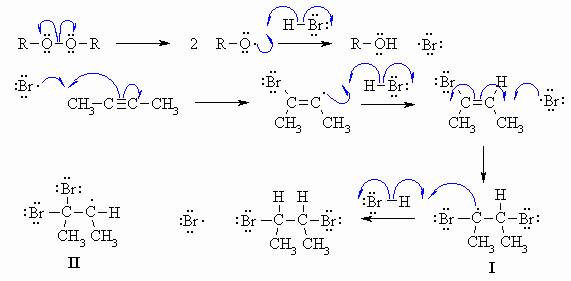 The weak O-O bond is broken
homolytically to give two alkoxy radicals that
react with H-Br generating the electrophilic Br radical. This sequence
provides the initiation steps. Then the Br radical adds to the alkyne
giving
a bromovinyl radical which then reacts with more H-Br forming the
stronger
C-H bond to give the bromoalkene and a new Br radical that can keep the
process going. When the Br radical reacts with the bromoalkene,
the
selectivity favours the formation of the radical I
where the electrons
of the Br already in place can stabilise the new radical (in the same
way
as Br stabilises the C+ formed in the addition of HBr to alkynes under
heterolytic conditions). The alternative regioselectivity gives
radical
II which is less stable. This new radical I
reacts with another
molecule of H-Br to give the vicinal dibromide and another Br radical
that
can keep the process going. Note that the selectivity of the reaction
is
different to that of the addition of H+ and Br- to the alkyne because
in
the radical process the Br radical is the electrophile (and not H+) and
so it is Br that adds first to the C=C.
The weak O-O bond is broken
homolytically to give two alkoxy radicals that
react with H-Br generating the electrophilic Br radical. This sequence
provides the initiation steps. Then the Br radical adds to the alkyne
giving
a bromovinyl radical which then reacts with more H-Br forming the
stronger
C-H bond to give the bromoalkene and a new Br radical that can keep the
process going. When the Br radical reacts with the bromoalkene,
the
selectivity favours the formation of the radical I
where the electrons
of the Br already in place can stabilise the new radical (in the same
way
as Br stabilises the C+ formed in the addition of HBr to alkynes under
heterolytic conditions). The alternative regioselectivity gives
radical
II which is less stable. This new radical I
reacts with another
molecule of H-Br to give the vicinal dibromide and another Br radical
that
can keep the process going. Note that the selectivity of the reaction
is
different to that of the addition of H+ and Br- to the alkyne because
in
the radical process the Br radical is the electrophile (and not H+) and
so it is Br that adds first to the C=C.
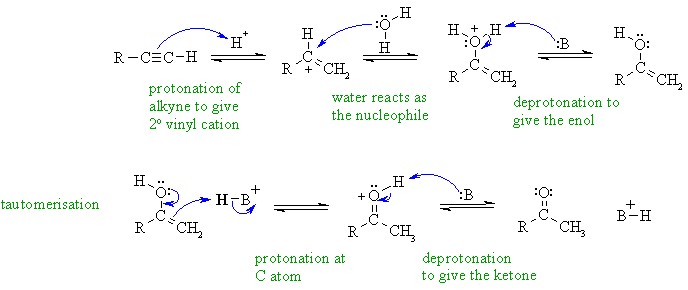
B
Hydration of an alkyne gives the
Markovnikov product, which is the
more highly substituted enol via the more stable secondary vinyl cation
which then undergoes acid catalysed tautomerism to the ketone. Notice
that
the proton donor is the acid catalyst and the nucleophile is water (no
hydroxide under acidic conditions).
C
Alcohols resemble water in many ways.
The reaction here is like the
hydration of an alkene except that Nu is now the ROH rather than H2O.
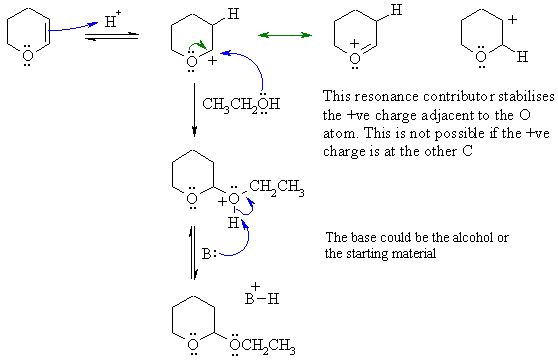 First protonate the C=C with the acid
catalyst (the ROH is not acidic enought
to do this on its own) to give the more stable C+. Note the O
atom
can donate its electrons to stabilise the C+ in a resonance
interaction.
Then the alcohol reacts as the Nu via the O lone pairs (just like water
would) to give an oxonium ion that would be then deprotonate to give
the
desired product and regenerating the H+ catalyst.
First protonate the C=C with the acid
catalyst (the ROH is not acidic enought
to do this on its own) to give the more stable C+. Note the O
atom
can donate its electrons to stabilise the C+ in a resonance
interaction.
Then the alcohol reacts as the Nu via the O lone pairs (just like water
would) to give an oxonium ion that would be then deprotonate to give
the
desired product and regenerating the H+ catalyst.
![[Chem 350 Home]](../mol.gif) Return
to
Homepage
Return
to
Homepage



