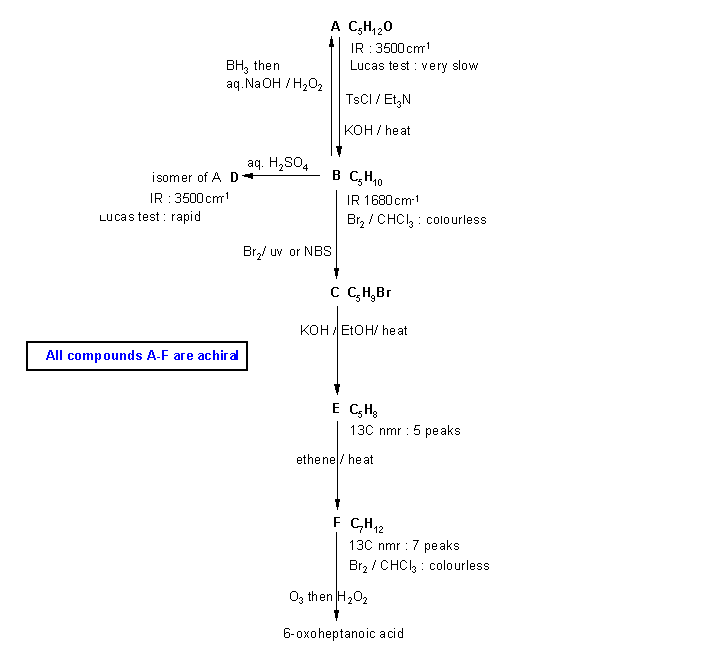
Note that these types of questions are often the most difficult on the examination. This is because they are holoistic in that they require a good understanding of the reactions and the concepts and the ability to apply those concepts. They can't be memorised, they require understanding and ability to apply. Hence they help discriminate those who truelly understand from those who have memorised and will soon forget.
A full schematic of the full solution is
presented below. This is
also presented in
several parts, where each diagram has more information added to it.
The idea behind this is to encourage students to see how to work
towards the answer by building up the answer from the information in
the question gradually.
Diagram
1 just gives all the
information from the question in black with no interpretation.
You can use this to try to solve the problem based on that information.

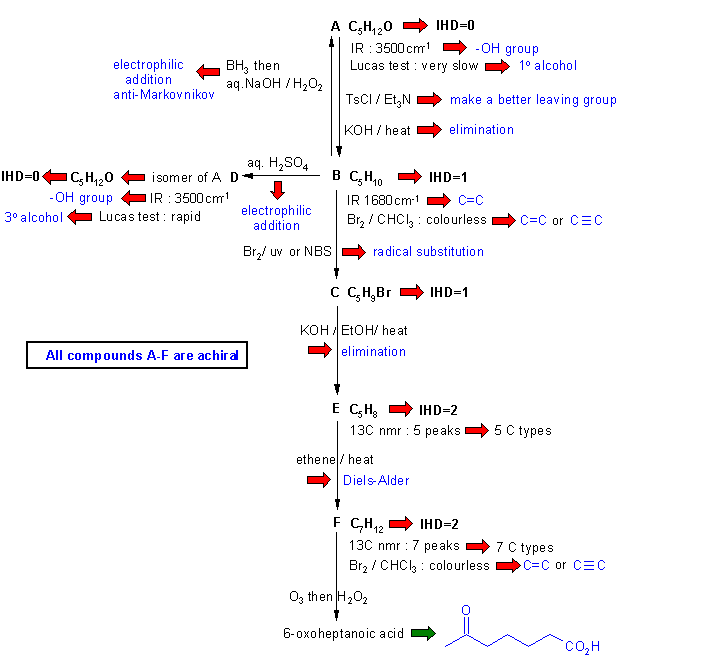
Diagram
2 gives the basic
information from the
question in black and deductions directly from this information are
indicated by red arrows and with blue text.
Diagram
3 is diagram 2 plus it
identifies points that
provide potential key information by
green arrows and key initial structures
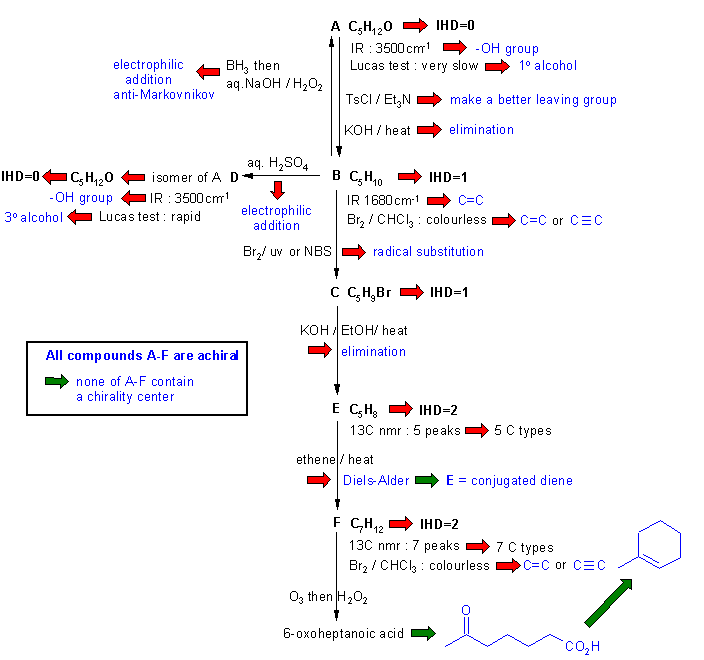
Diagram
4 gives the
completed solution with the structures linked via the blue
arrows to show the path required to work them all out. There are, of
course,
other possible thought pathways.
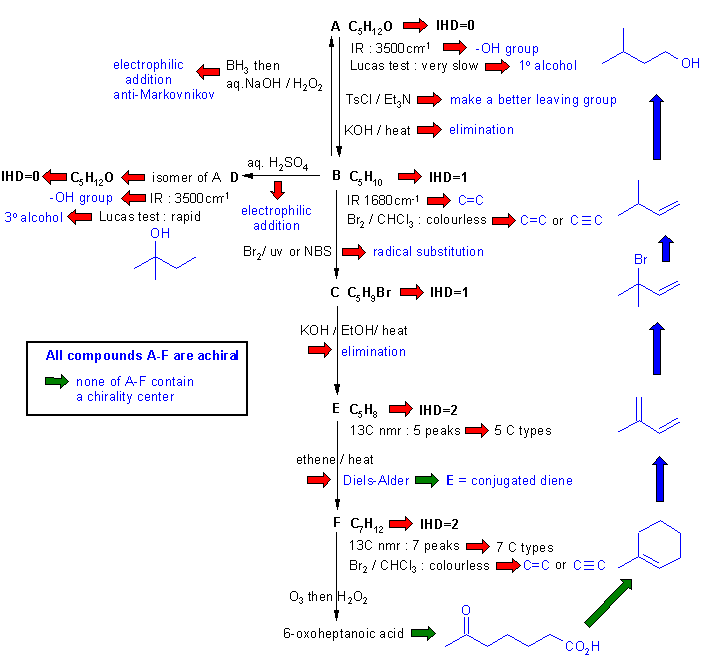
Note that the structure of F
is perhaps the pivotal point. We should be able to deduce that it's a
cyclohexene with a methyl group. The key point to realise is that in
order for F to be achiral, then
the methyl group must be at C1. Hence E
must be 2-methyl-1,3-butadiene. Other important points are to realise
that A is a primary alcohol, B is a terminal alkene and the D is a tertiary alcohol.
mechanisms
to follow