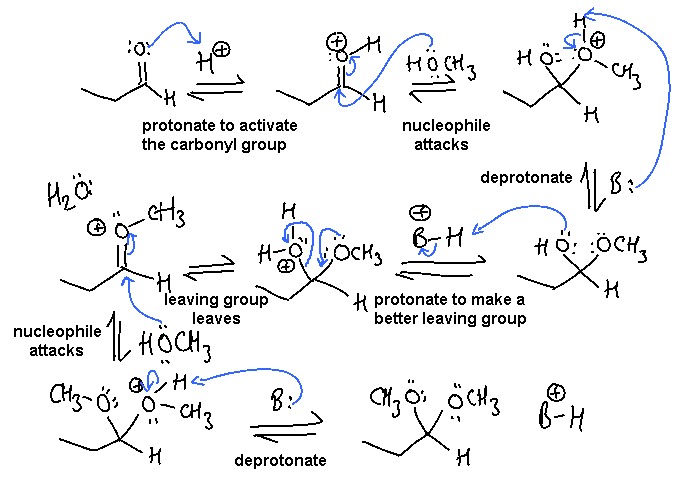
In this scheme, the base, B:, could be C=O, R-OH or the conjugate base of the acid catalyst
Note
that no other reagents are needed in order to complete any of these sequences,
you should only be using what is there.
Part A:
i. Conversion of an aldehyde to an acetal : nucleophilic addition to
a carbonyl followed by nucleophilic substitution of an alcohol:

In this scheme, the base, B:, could be C=O, R-OH or the conjugate base of the acid catalyst
ii. Nitration of benzene, an electrophilic aromatic substitution:
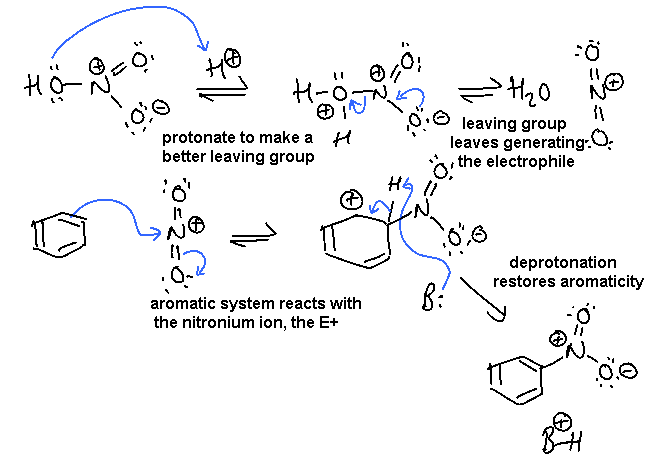
In this scheme, the base, B:, could be -OH or the conjugate base of the acid catalyst
iii. Grignard reaction of a carboxylic acid derivative, a nucleophilic substitution followed by a nucleophilic addition:
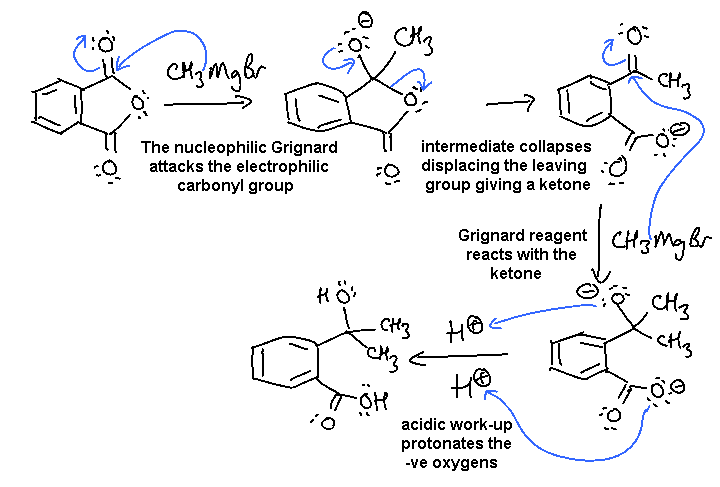
Part B:
i. The key issue is to focus on the new 5 membered ring formed adjacent
to the ester by a double alkylation via the reaction of an alkyl bromide and
an epoxide as the electrophile (note: it could also be drawn as reaction of
the epoxide followed by reaction of the alkyl bromide).
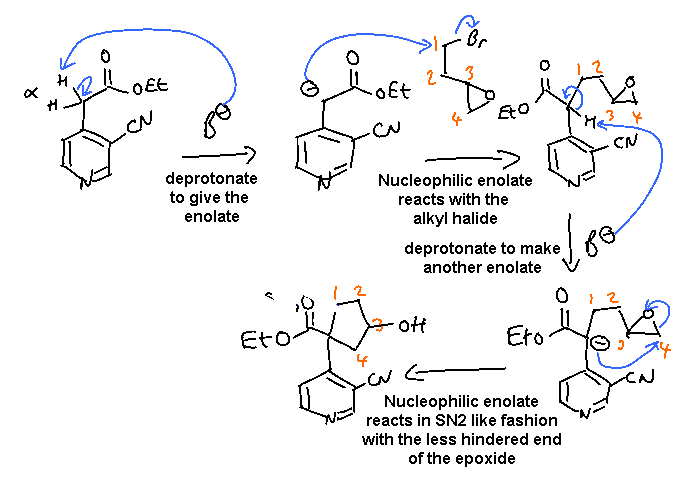
In this scheme, the base, B-, was EtO-
ii This example combines electrophilic addition to an alkene that leads to a carbocation that is then involved in electrophilic aromatic substitution.
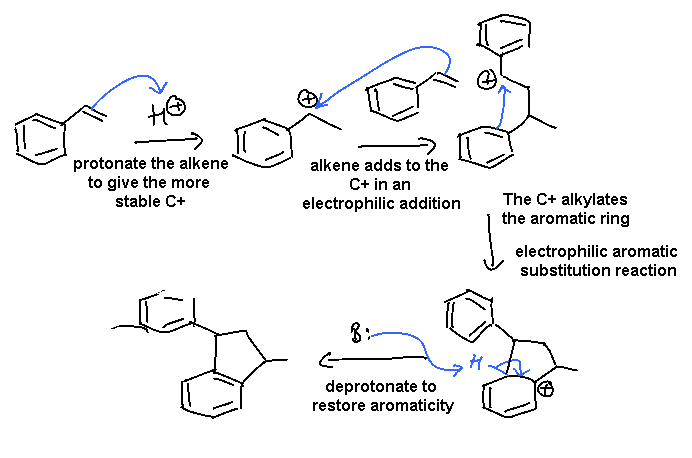
In this scheme, the base, B:, could be C=C, or the conjugate base of the acid catalyst