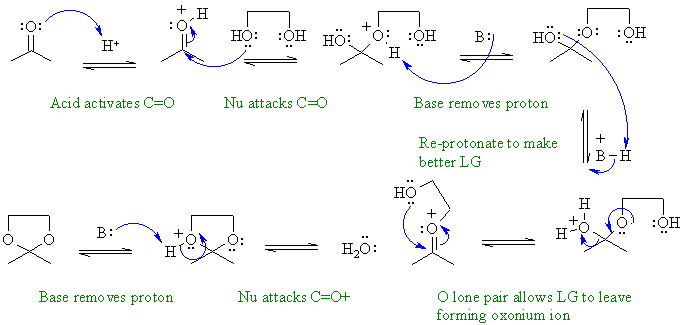
B: = C=O groups, H2O, ROH groups, conjugate base of the acid used
Note that no other reagents are needed in order to complete any of these sequences, you should only be using what is there.
Part A:
i. Reaction of a ketone with a 1,2-diol alcohol to form a cyclic ketal.

B: = C=O groups, H2O, ROH groups, conjugate base of the acid used
ii. Decarboxylation of a beta-ketoacid under acidic conditions. The loss of the CO2 gives the enol which then undergoes acid catalysed tautomerisation to the ketone.

Part B:
i. The change in the hydrocarbon skeleton indicates a carbocation rearrangement:
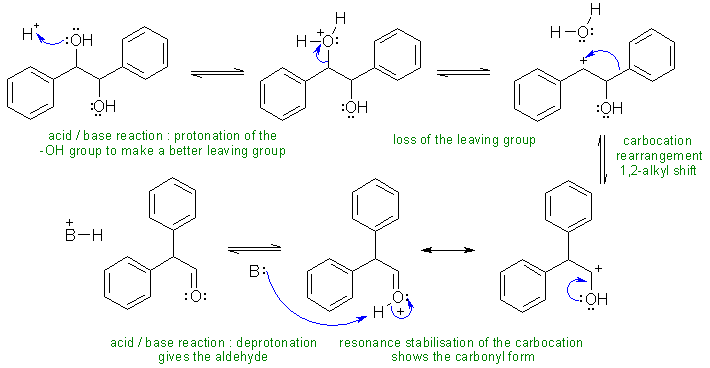
B: = H2O, conjugate base of the acid used, -OH groups
ii . An example of a nitro enolate :
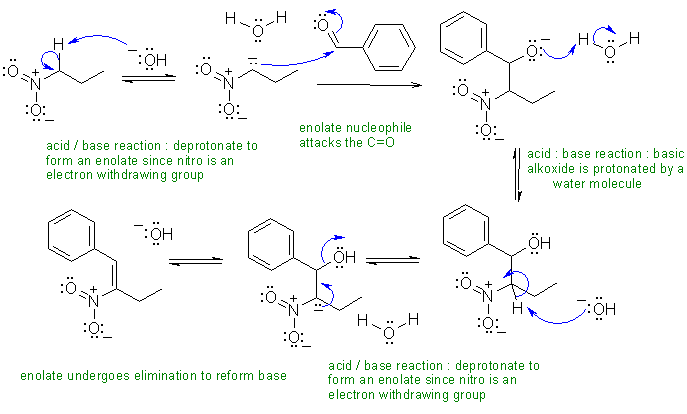
Common general errors: (1) Ignoring reaction conditions i.e. effect of acidic or basic environment (2) poorly drawn arrows, e.g. not starting at the Nu site (3) backwards arrows (4) wrong use of arrows e.g. resonance (5) not showing formal charges (6) missing arrows esp. when adding or removing H+ (7) compressing several mechanistic steps into a single step