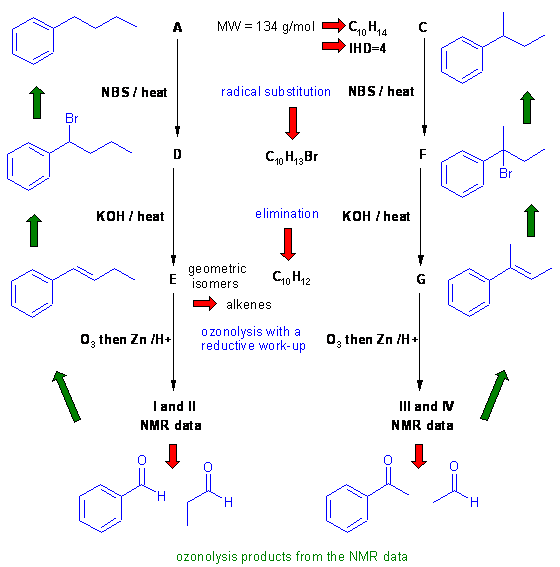
A schematic of the solution is shown below. The information from the question is given in black. Deductions directly from this information are given in red. Points that provide potential key information are shown in green which leads to the structures which are linked via the blue arrows to show the path required to work them all out. There are, of course, other possible thought pathways. In this question, some key information can be worked out before looking at the reactions themselves.
Since A, B and C are isomeric hydrocarbons with MW=134.2 g/mol we can work out the MF = C10H14 this has an IHD = 4 which is quite high for a low MW and suggests a benzene system.
The reactions of A-C with hot acidic permanganate and NBS suggest reactions of alkyl substituents of aromatic systems. Note that because B does not react under either of these sets of conditions, it suggests B is t-butylbenzene.
Spectral data:
H NMR 9-10 ppm suggests an aldehyde. 13 C NMR peaks 190-220 suggest C=O in aldehydes and ketones.
Once you work out I-IV, it's a question of reassembling the C=O to obtain the alkene starting materials and then working back through the reactions.
![[Home]](mol.gif)