
Note
that no other reagents are needed in order to complete any of these sequences,
you should only be using what is there.
A1
Bromine react with alkenes via an electrophilic addition mechanism, via a cyclic bromonium ion intermediate which is then opened by the attack of a nucleophilic species, the bromide ion. This attack of the nucleophile normally results in an overall anti addition of the two bromine atoms as is seen here with cyclopentene (for simplicity):

Common errors: The process is not a radical reaction nor does it proceed via a carbocation. In order to avoid these kinds of errors one needs to have a better grasp of (1) the specifics of reagents and (2) the various mechanisms. It's not like this is an uncommon or rare reaction in this course.
A2
This is a based on the laboratory experiment on the hydrolysis of sucrose using aq. acid. We are looking at the ring opening of glucose. Protonation of the O adjacent to the anomeric center creates a better leaving group. The group is made to leaving by breaking the bond to the anomeric carbon because this leads to a resonance stabilised carbocation. Looking at the stablised form, simple removal of the proton reveals the aldehyde functional group.

B1
Bromine react with alkenes via an
electrophilic addition mechanism,
via a cyclic bromonium ion intermediate which is then opened by the
attack
of a nucleophilic species. This attack of the nucleophile normally
results
in an overall anti addition of the two bromine atoms as is seen here
with
2-butene.
However, Z-1-phenylpropene is not as
stereoselective. In this
case, the bromonium ion can open to give a very stable resonance
stabilised
carbocation. This planar carbocation could be attacked on either face
by
the bromide ion and so is not stereoselective. The 73% selectivity for
anti indicates that these two pathways are competing with each other. Answering this problem requires critical thinking and applying concepts from the course to a new situation.
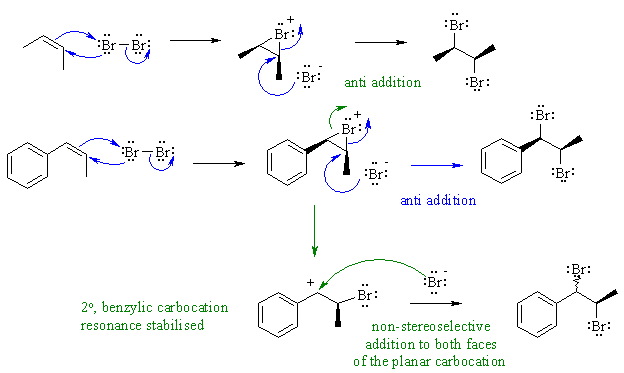
Common errors: The process is not a radical reaction. Sterics can not explain the syn addition, they would cause the bromide ion to attack the methyl of the cyclic bromonium ion not the opposite face where the large bromine atom blocks the way in the cyclic bromonium ion intermediate.
B2
The reaction is hydroboration / oxidation. This was discussed as a way to hydrate alkenes in an anti-Markovnikov manner. Here though we have an alkyne. We discussed the hydration of alkynes using aq. H2SO4 / Hg2+ as a way to give ketones via a Markovnikov addition to give an enol that tautomerises to give the ketone. In this question we need to bring those known reactions together. Hydroboration / oxidation of the alkyne should then give the anti-Markovnikov enol.... this should then tautomerise to give the aldehyde. Answering this problem requires critical thinking and applying concepts from the course to a new situation.
