Note that these types of questions are often the most difficult on the examination. This is because they are holistic in that they require a good understanding of the reactions and the concepts and the ability to apply those concepts. They can't be memorised, they require understanding and ability to apply. Hence they help discriminate those who truly understand from those who have memorised and will soon forget.
A schematic of the solution is shown below. The information from the question is given in black. Deductions directly from this information are given in red. Points that provide potential key information are shown in green which leads to the structures which are linked via the blue arrows to show the path required to work them all out. There are, of course, other possible thought pathways.
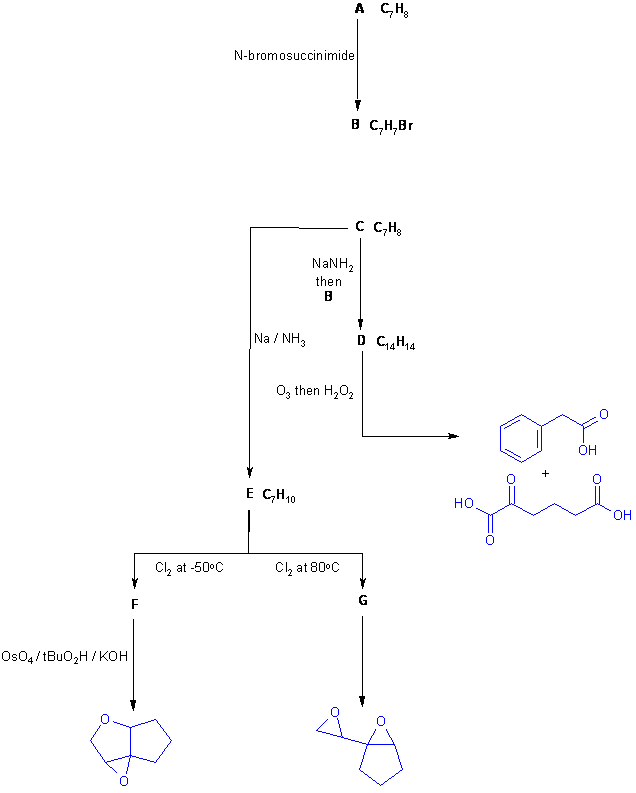
Diagram 1 just gives all the information from the question in black with no interpretation. You can use this flow chart to try to solve the problem. Drawing a flow chart is a key step in developing the skills to solve these types of questions.

Diagram 2 gives the basic information from the question in black and deductions directly from this information are indicated by red arrows and with blue text plus it identifies the functional groups and points that provide potential key information by green arrows and key initial structures.
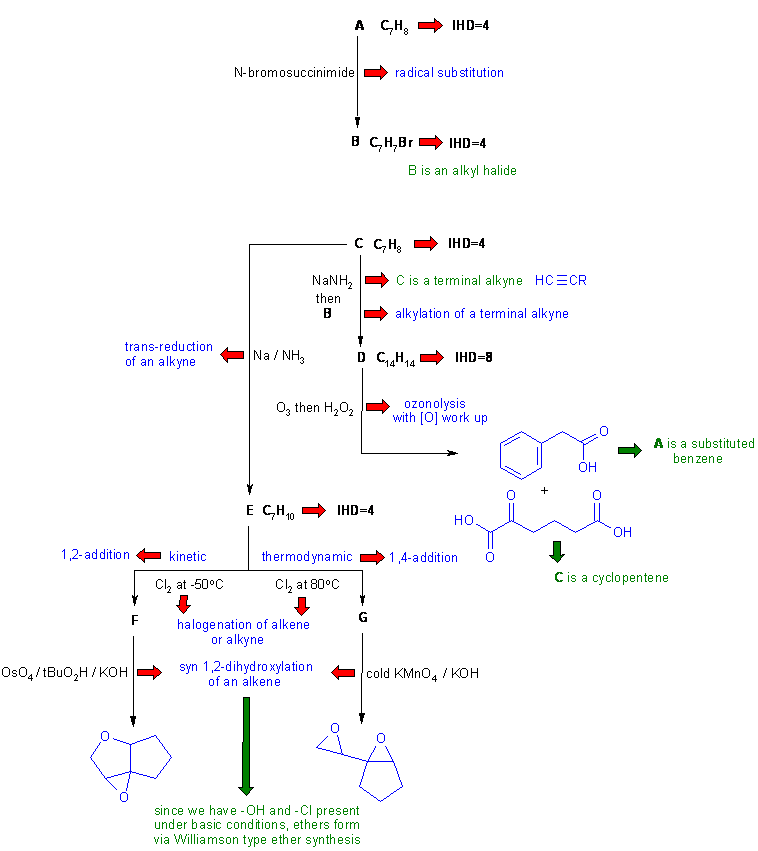
Diagram 3 gives the completed solution with the structures in blue. Key steps are the structure of the final product (based on the name and / or spectroscopic data) and the conjugated diene nature of C.
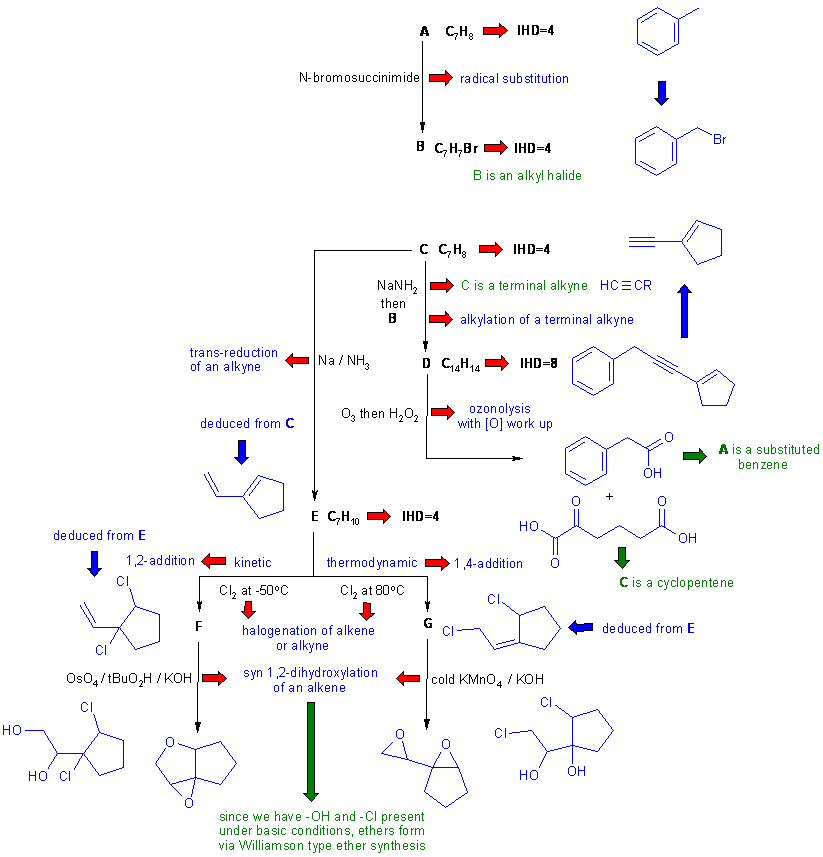
The crux to this problem is recognising the cyclopentene system from the ozonolysis date where both an alkyne and an alkene has been cleaved - this was indicated by the presence of the 4 C=O groups in the two products.
Common errors:
1. missing the benzylic carbon after the acetylide ion SN2 reaction with benzyl bromide (C + B).
2. Not understanding the difference between the kinetic and thermodynamic products.
3. While most students got the 1,2 addition product F, the 1,4 addition product was often missing the migration of the double bond.
4. G was often given as the alternate 1,2 addition product which can't explain the formation of the diepoxide.