Answers to the synthesis problems are given below. These answers have been selected as they are short and efficient, but there are probably other reasonable solutions. Note some targets have specific stereochemistry that needs to be considered.
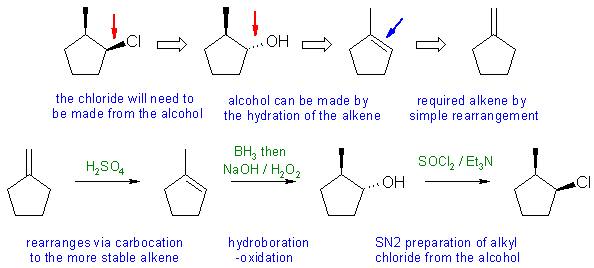
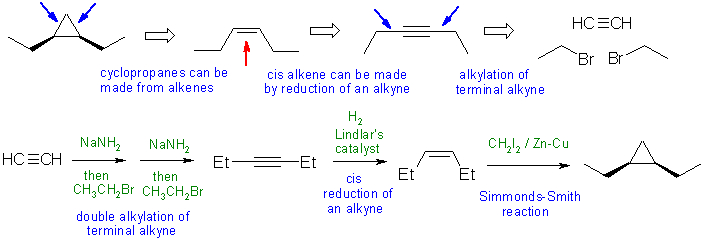
When planning syntheses, one should try to work backwards by functional group analysis. The diagrams show the "retrosynthesis" - the design or plan and then below that the reaction scheme step-by-step (as required in the question)

Notes: Unlike HBr, HCl does not undergo a radical addition with anti-Markovnikov to alkenes. Addition of HCl to 1-methylcyclopentene will give 1-chloro-1-methylcyclopentane. Need to pay attention to the stereochemistry and avoid SN1 reactions.
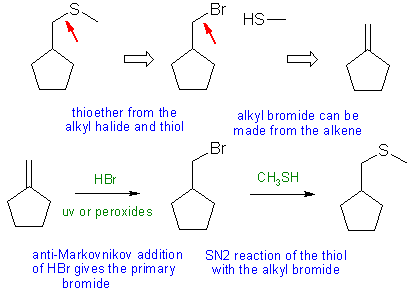
Notes: the shortest one of the set, but the least attempts! Thioether made using Williamson ether type synthesis.
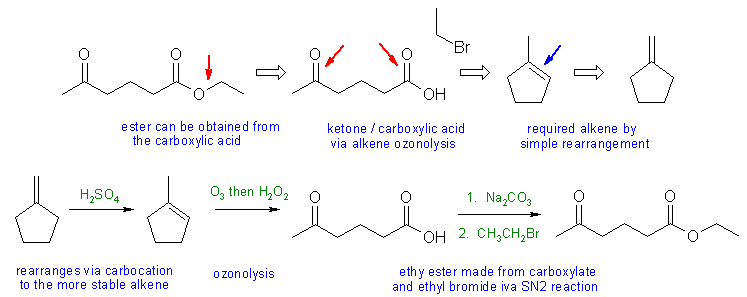
Notes: the most common mistakes were in making the ester.... if use an alkyl halide, then you need a suitable base to make the carboxylate nucleophile. Poor choices of base were common e.g. use of Na or NaNH2. If you use an alcohol, then an acid catalyst is needed to activate the carbonyl group and the leaving group.
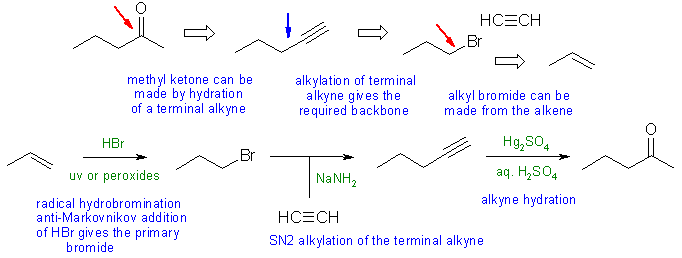
Notes: the most common problems were poor control of the regiochemistry by using an internal alkyne rather than a terminal alkyne or trying to make 1-bromopropane using radical halogenation of propane (the major product would be 2-bromopropane (96% yield)) rather than from propene.
General comments.... Lots of students made more use of Chem 351 reactions over 353 reactions. It's important to note that the 353 materials are often more selective and therefore give better control than the 351 reactions students elected to use. In addition, the question allowed students to start from compounds with 3 C or less. Lots of students started from hydrocarbons with 3 C or less and then made errors in making the materials they could have just used as starting materials.