
Note
that no other reagents are needed in order to complete any of these sequences,
you should only be using what is there.
General common errors:
(1) incorrect formal charges (2) backwards arrows (3) not showing the arrows for all the bonding changes (4) misuse of resonance / equilibrium arrows (5) vague arrows e.g. not starting on a bond or lone pair.
A1
Alkene hydration to an alcohol. Aqueous acid reacts with alkenes via an electrophilic addition mechanism, via a carbocation intermediate which is then attacked by a nucleophilic species, the water molecule. In the scheme, the base, B:, could be the alkene, water or HSO4-.

Common errors: (1) Using B: to represent a base in a reaction but then not listing what B: could be based on species that are present in the reaction mixture. (2) Using HO- as the nucleophile in an acidic medium.
A2
This is a transesterification under basic conditions (from the biodiesel laboratory experiment) where the methanol is deprotonated by the hydroxide to form the reactive nucleophile methoxide that attacks the electrophilic C in the ester carbonyl group, the tetrahedral intermediate collapses to lose the other alcohol group as the leaving group. This is then protonated in the work-up. In the scheme below R and R' have been used to simplify the drawing.
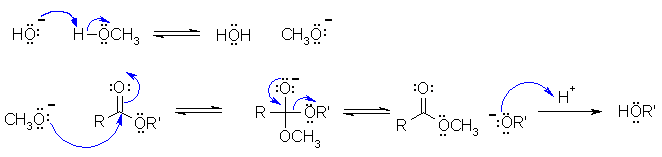
B1
HBr in the presence of uv light will be a radical addition type process with a radical chain type process. This means it needs to be drawn with single headed arrows indicating the motion of individual electrons. Note that the question indicates that you should be considering an excess of HBr. The major product turns out to be the 1,2-dibromide for reasons explained below.
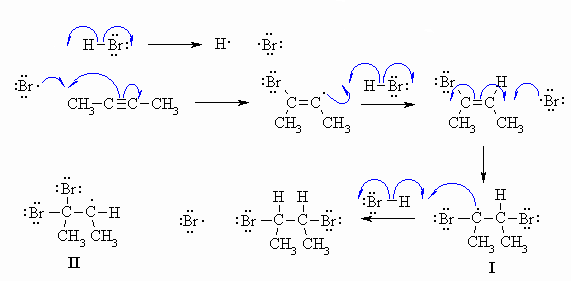
Common errors: (1) The use of uv implies that ths is a radical reaction. (2) Showing a heterolytic process using double headed arrows instead of a radical process using single headed arrows. (3) Using a peroxide (the question does not include peroxide and clearly states that no other reagents are required). (4) Adding the H radical to the pi bond first (it's the Br radical that adds first). (5) Not rationalising the selectivity in terms of intermediate stability. (6) Not using a radical chain type process. (7) Forming diradicals.
B2
Chlorination of a conjugated diene at higher temperatures favours conjugate or 1,4-addition. There are a couple of reasonable mechanistic pathways: (1) addition of the Cl to give an allylic carbocation or (2) via a chloronium ion that then ring opens.
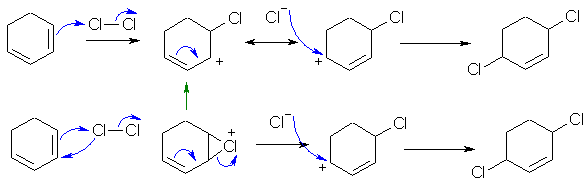
Common errors: (1) The process is not a radical reaction. (2) Showing the 1,2- addition product. (3) Not saying why the 1,4-addition product is favoured. (4) using the wrong arrows (5) arrows that did not reflect the bonding changes drawn.