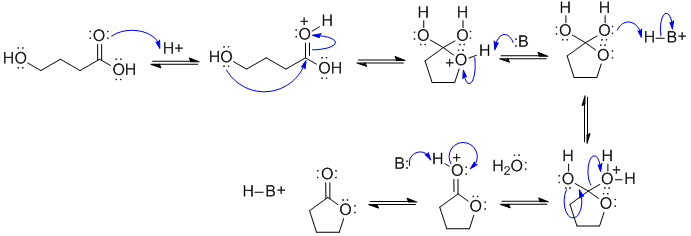
Note that no other reagents are needed in order to complete any of these sequences, you should only be using what is there.
A1
This is an example of the Fischer esterification (just an intramolecular example to give a cylic ester or lactone)

In this scheme, B: could be the conjugate base of the acid catalyst, C=O, or OH groups.
A2
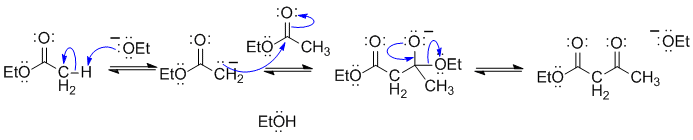
This is the most common example of a Claisen condensation.
B1
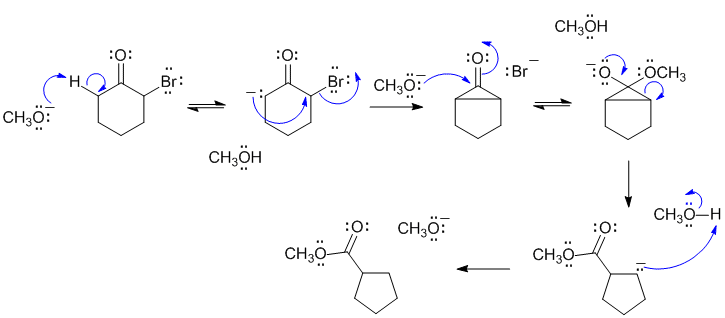
A Favorskii rearrangement. Use the base to form the enolate at the alpha-H on the left as drawn, intramolecular alkylation gives the cyclopropanone intermediate that was identified in the question. Attack of the methoxide as a nucleophile on the ketone followed by reformation of the carbonyl and breaking the ring strained cyclopropanone gives the ester and a carbanion which just needs to be protonated.
B2
This is the reagent used to protect an alcohol as a THP ether (an acetal). View it as a reaction of an alkene. Protonate the C=C so that the +ve charge is adjacent to the O (it's resonance stabilised there), now add the ROH (as the Nu) to the carbocation, and remove the proton to give the acetal.
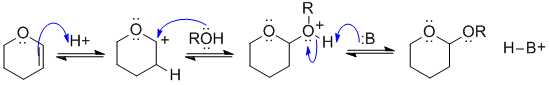
In this scheme, B: could be the conjugate base of the acid catalyst, C=C, or ROH groups.
Common general errors: (1) Ignoring reaction conditions i.e. effect of acidic or basic environment (2) poorly drawn arrows, e.g. not starting at the Nu site (3) backwards arrows (4) wrong use of arrows e.g. resonance (5) not showing formal charges (6) missing arrows especially. when adding or removing H+ (7) compressing several mechanistic steps into a single step (8) drawing primary carbocations (no need to, avoid them, there are too unstable) (9) not knowing the basic mechanism types.