Note that these types of questions are often the most difficult on the examination. This is because they are holistic in that they require a good understanding of the reactions and the concepts and the ability to apply those concepts. They can't be memorised, they require understanding and ability to apply. Hence they help discriminate those who truly understand from those who have memorised and will soon forget.
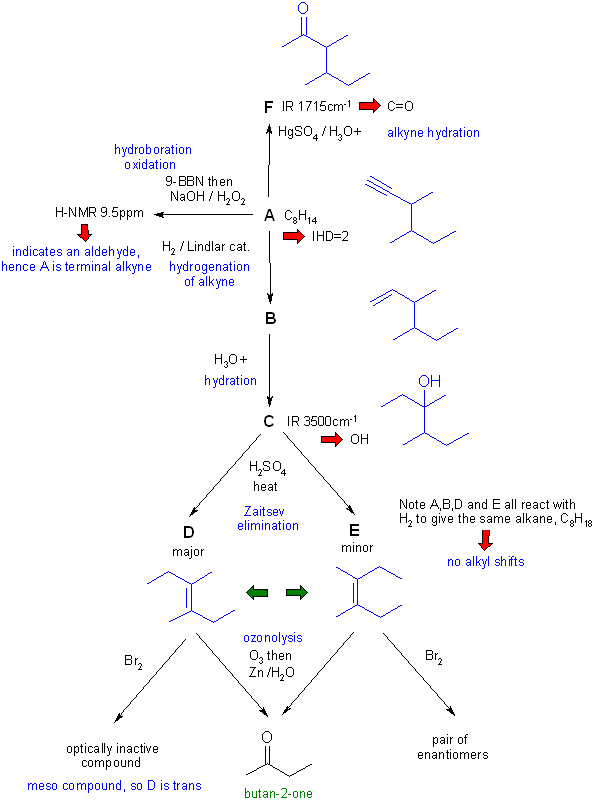
A schematic of the solution is shown below. The information from the question is given in black. Deductions directly from this information are given in red. Points that provide potential key information are shown in green which leads to the structures.... the path is D/E to C then A/B and then F to work them all out. There are, of course, other possible thought pathways.

D = trans-3,4-dimethylhex-3-ene or (E)-3,4-dimethylhex-3-ene
The product of the reaction of D with Br2 is because the product is a meso-compound resulting from the anti-addition of the bromine to the trans -alkene.
The crux to this problem was the ozonolysis of D and E giving only butan-2-one. This means that D and E are symmetrical alkenes and stereooisomers. Since D is the major product, it should be the trans isomer. This is confirmed by the stereochemical information about the reactions with bromine. So E is the cis isomer.
Other important steps are :
1. Hydroboration oxidation of A gives an aldehyde therefore A is a terminal alkyne.
2. Since A is a terminal alkyne, B is a terminal alkene and F is a methyl ketone.
3. Rearrangement via a hydride shift to get alcohol C
Common mistakes: