Part
7: LABORATORY
Experimental yields.... First you need to write out and balance the reaction equation and then work out the moles of each reagent used to determine the limiting reagent. You need to remember that TsOH is just a catalyst (check the balanced equation).
| |
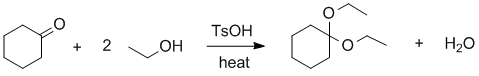
C6H10O + 2 C2H5OH --> C6H10(OCH2CH3)2 + H2O
|
| MW (g/mol) |
98.145 |
46.06 |
172.26 |
| amount (g) |
9.815 |
10mL x 0.789g/mL |
10.00 |
| mmoles |
100 |
170 |
58 |
| mmoles/ coefficient |
100 / 1 |
170 / 2 = 85 |
58 / 1 = 58
|
Therefore, in the last row of the table where the stoichiometric coefficient from the balanced equation is divided into the number of moles, we can see that the limiting reagent is the ethanol. This means that the maximum amount of product that can be formed is 85 mmol (based on the reaction stoichiometry). Hence the % yield, based on obtained / max. possible = 58 / 85 = 68 %.
Common general errors: (1) not balancing the reaction equation, (2) not determining the molecular formulae or weight correctly (3) not determining the limiting reagent correctly and (4) not knowing how the calculate an experimental yield. Considering the number of times yields are calculated in the laboratory during the semester (year) and similar questions on past finals, some students still can't do this high school level task.
Note that it doesn't matter whether you work with grams or moles of product the answer should be the same.
![[Organic Home]](../353w10/mol.gif) Return to Homepage
Return to Homepage

![[Organic Home]](../353w10/mol.gif) Return to Homepage
Return to Homepage