Part 5: MECHANISMS
Note that no other reagents are needed in order to complete any of these sequences, you should only be using what is there.
General common errors:
(1) incorrect formal charges (2) backwards arrows (3) not showing the arrows for all the bonding changes (4) misuse of resonance / equilibrium arrows (5) vague arrows e.g. not starting on a bond or lone pair.
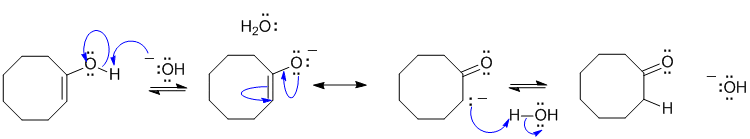
A1 Enol / ketone tautomerisation in basic medium:

Common errors:
A2 Acid catalysed hydration of an alkene where a carbocation rearrangement is involved. In the scheme below the acid is simply shown as H+ to simplify the picture. The acid protonates the C=C to give a secondary carbocation. A 1,2-hydride shift produces the more stable tertiary carbocation which then attacked by water as the nucleophile. A base then removes a proton to produce the alcohol. The possible bases that are present in the reaction mixture are indicated below the scheme:
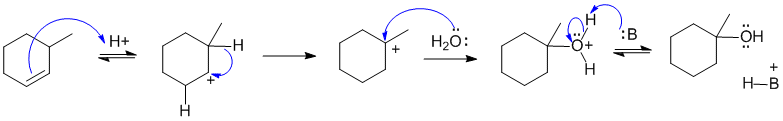
B: = alkene, HSO4-, or H2O
Common errors:
B1 Opening of an unsymmetrical epoxide with a neutral nucleophile under acidic conditions. Activate the epoxide by protonation by the acid catalyst then attack of the neutral O as the nucleophile at the more substituted carbon from the opposite face to the O sets up the stereochemistry. Deprotonation gives the methoxy alcohol product:
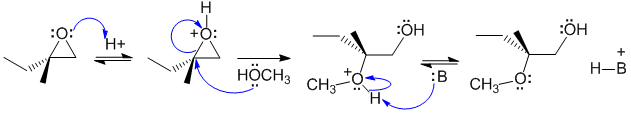
B: = epoxide, A-, or ROH
Common errors:
B2 The alkene reacts with the chlorine to generate the cyclic halonium ion and a chloride ion. Attack attack of the neutral O as the nucleophile at the more substituted carbon from the opposite face to the Cl sets up the stereochemistry. Deprotonation gives the methoxy chloro product:
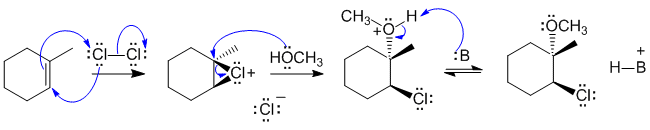
B: = Cl- or ROH
Common errors: