Note that no other reagents are needed in order to complete any of these sequences, you should only be using what is there.
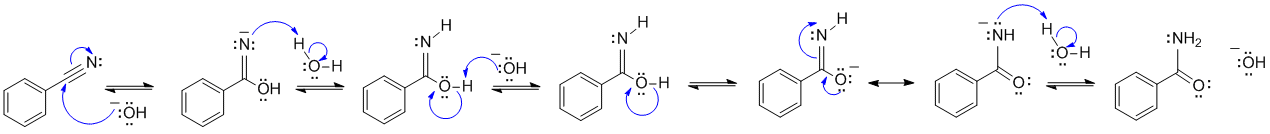
A1
This is an example of nitrile hydrolysis under basic conditions:

Common errors: showing steps more appropriate for acidic conditions
A2
The Aldol condensation.
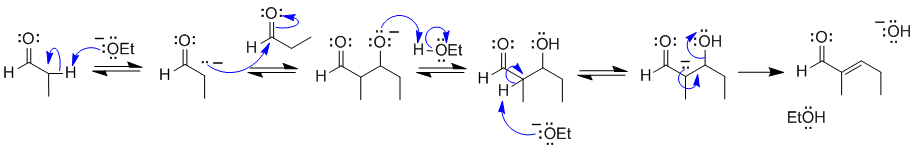
Common errors: deprotonating at the wrong position, not counting C atoms
B1
Grigard reagent reacting with a carbonate ester. This is very much like the reaction of a Grignard with a regular ester, except there are now 2 leaving groups and therefore the Grignard reagent can react with 3 equivalents of the Grignard reagent to give the tertiary alcohol:
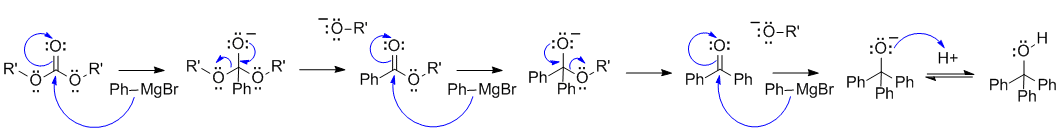
Common errors: acidic species in the presence of the very basic Grignard reagent, incorrect conversion of a ketone to the alcohol.
B2
This is the reagent used to protect an alcohol as a THP ether (an acetal). View it as a reaction of an alkene. Protonate the C=C so that the +ve charge is adjacent to the O (it's resonance stabilised there), now add the ROH (as the Nu) to the carbocation, and remove the proton to give the acetal.
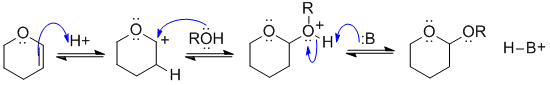
In this scheme, B: could be the conjugate base of the acid catalyst, C=C, or ROH groups.
Common errors: formation of primary C+
Common general errors: (1) Ignoring reaction conditions i.e. effect of acidic or basic environment (2) poorly drawn arrows, e.g.vague (i.e. starting / ending "in space" (3) backwards arrows (4) wrong use of arrows e.g. resonance (5) not showing formal charges (6) missing arrows especially when adding or removing H+ (7) compressing several mechanistic steps into a single step (8) not knowing the basic mechanism types (9) drawing arrows that were inconsistent with the resulting bonding changes.